The development of biomaterial-based vaccine platforms against viruses
Posted: 31 March 2021 | Dr Kaushik Chatterjee (Indian Institute of Science), Dr Sonal Asthana (Indian Institute of Science), Dr Sushma Kumari (Indian Institute of Science) | No comments yet
Recent years have seen an increase in the development of biomaterial and nanoparticle-based vaccine formulations. Sushma Kumari, Sonal Asthana and Kaushik Chatterjee from the Department of Materials Engineering at the Indian Institute of Science discuss why these materials have such high potential in the fight against infectious diseases.

Abstract
The scientific community’s response to the COVID-19 pandemic has seen a shift of research focus towards the development of different approaches and methods to fight against infectious diseases. Novel biomaterials and nanotechnology-based vaccine formulations will play an important role in new strategies to contain viral pandemics. This article highlights the current efforts and opportunities offered by biomaterials and nanomaterials in the development of vaccines to prevent the spread of contagious diseases and tackle future outbreaks.
The importance of vaccines
Humans have recently been exposed to several life‑threatening contagious diseases, such as outbreaks of Zika, Ebola, Middle East Respiratory Syndrome (MERS), Severe Acute Respiratory Syndrome (SARS) and most recently Severe Acute Respiratory Syndrome coronavirus 2 (SARS‑CoV-2). Vaccines and immunisation are the most effective way of protecting humans against highly infectious viruses. However, existing vaccines are ineffective against new viral strains and lack the desired prophylactic measure against infectious diseases. Therefore, continuous research is ongoing to produce vaccine formulations by developing effective antigen, adjuvants as immune enhancers and vaccine delivery systems.
Biomaterials to enhance vaccine formulations
Continuous research is ongoing to produce vaccine formulations”
The design of a virus-mimicking immunogenic substance to elicit an immune response is the holy grail in vaccine development. In recent years, biomaterials encompassing biopolymers, self‑assembled systems, inorganic materials and synthetically engineered cells have been used to engineer virus-mimicking materials for next‑generation vaccines and some of the potential candidates are currently in clinical trials.1,2,3 Considering the diversity of biomaterial-based vaccine platforms, particles, virus-like particles (VLPs), liposomes, scaffolds and microneedles are the most promising vaccine delivery platforms that have been developed and have gained significant attention because of their ability to stimulate a strong immune response against a pathogen.4 Biomaterials have several advantages for use in vaccines including adjustable properties of size, shape, morphology, surface functionality, charge, degradation and control over release kinetics. Biomaterials are also represented as potential adjuvant materials in vaccine formulations, which increases the antigenicity of an antigen and allows a single dose of vaccination to elicit an immune response without requiring a booster shot.5 Additionally, conjugating biomaterials with ligands for dendritic cells (DCs) allows specific binding to receptors on an immune cell and reduces the local toxicity and adverse reactions.
Nanomaterials to enhance the development of vaccines
A combination of nanotechnology and biomaterials provides an appropriate vaccine carrier system that enhances the delivery of inoculations.2,6 The key advantages of engineered nanomaterials in vaccine development include the protection of antigen within the nanoparticle, interaction with specific immune cells, targeted immunogen delivery to the antigen-presenting cells (APCs) and production of appropriate humoral and cellular immune responses.7 Nanoparticle-based vaccine delivery systems have several other merits, for example:
- high payload capacity
- high stability at extreme pH
- ability to facilitate the encapsulation of both hydrophilic and hydrophobic molecules
- adjuvant properties
- efficient cellular uptake
- bioavailability enhancement
- presentation of antigens in biomimetic formats for specific immune cell targeting.
In the past few years, we have seen the extensive exploration of nanoparticles from both organic and inorganic materials, self/assembled proteins and liposomes in vaccine delivery systems.8
Vaccines to prevent diseases
Over the past decade, several biomaterial and nanoparticle-based vaccine formulations have been proposed and successfully examined against infectious diseases.2,4 Importantly, many vaccines are in clinical trials at present and the most successful ones will be available to combat life‑threatening diseases. Biomaterials are promising candidates that could provide stability to the antigen/cargo, allowing for easy preservation and storage as well as simple and uniform dispersal of cargo in a sustained manner to optimise immune response. They can also act as adjuvants to potentiate specific and desirable immune responses.
For example, antigen-loaded poly(lactic-co-glycolic acid) (PLGA) particles have been studied for immunomodulatory properties of a vaccine against several diseases, such as hepatitis B, tuberculosis, malaria, allergy antigens and others.9 Efficacy of a polysaccharide-based AdvaxTM delta inulin adjuvant has been shown to enhance response against seasonal and pandemic viral infections such as hepatitis B, influenza, Flaviviruses, West Nile Virus (WNV), Murray Valley encephalitis virus (MVEV), SARS, human immunodeficiency virus and others.10 Many other organic and inorganic nanoparticle‑based systems have shown high immunogenicity and defence mechanisms against pathogens.
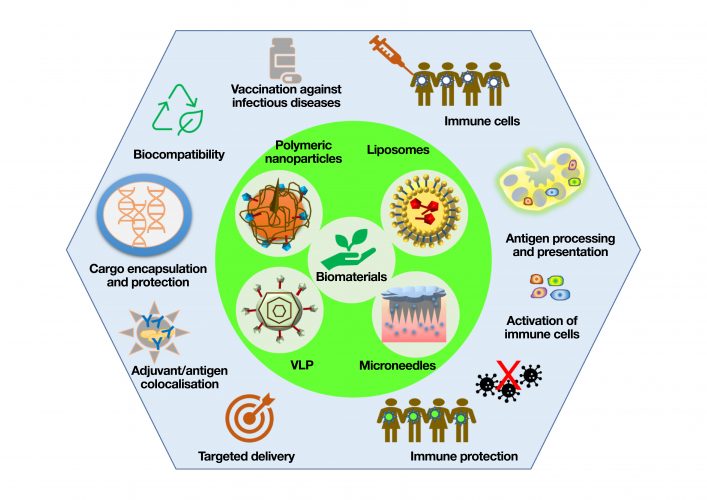

Figure 1: Biomaterial-based vaccine platforms such as polymeric nanoparticle, liposomes, VLPs and microneedles and their key advantages for developing the next generation of vaccines
Another class of nanoparticles, liposomes, prepared by self-assembly of biodegradable phospholipids with aqueous core, are the highest clinically-approved vaccine delivery systems. For example, liposome formulations integrating biomaterials such as Epaxal® virosomes against HAV infection, Inflexal™ V virosomes against influenza and interbilayer-crosslinked multilamellar vesicles (ICMVs) against hepatitis C, malaria and Ebola, have demonstrated successful immunogenicity.11,12 Liposomes are in high demand because they are highly effective at encapsulating both hydrophilic and hydrophobic drugs, enabling surface functionalisation and enhanced penetration of cells. Recently, lipid nanoparticles encapsulated with self‑amplifying RNA encoding the SARS-CoV-2 Spike (S) protein showed high immunogenicity by eliciting strong SARS-CoV-2 IgG antibody and robust cellular immune responses in mice.13 Preliminary data of these liposome-designed SARS-CoV-2 vaccines are promising and accelerate translation to the clinic.
Newly-developed biomaterial-based pain‑free microneedle technology has also shown great potential to deliver antigens into the skin, having extremely immune-reactive cells. Extensive research has been done to develop microneedle-based polio, influenza and hepatitis B vaccines.14 Recently, MERS‑CoV-S1 protein subunit trimer as an antigen has been delivered using a polymeric microneedle array‑based MERS vaccine and the pre-clinical results in mice are promising.15 Research is ongoing in the development of a similar microneedle vaccine based on SARS‑CoV-2 S protein subunit trimers.
Biomaterial-based adjuvants are well known to enhance the immunostimulatory properties of the antigen”
To fight cancer, the capabilities of biomaterial and nanomaterials-based cancer vaccines have been tested for models of leukaemia, lymphoma, melanoma and breast cancer, and reviewed in some recent articles.1,16 Here, nanocarriers such as PLGA nanoparticles, liposomes and polymeric scaffolds have been used to deliver the chemotherapeutics specifically to lymph nodes, spleen and tumour to reduce the systemic effects and safety concerns, to promote tumour‑specific T-cell responses and combat tumour growth.
Safety and efficacy of biomaterial‑based vaccines
Biomaterials and nanomaterial-based vaccine delivery systems enable the easy manipulation of antigen delivery rate with no additional booster and induce appropriate host immune responses with spatial and temporal accessibility of antigens to DCs of the immune system.1 Furthermore, biomaterial‑based adjuvants are well known to enhance the immunostimulatory properties of the antigen and are safe, biodegradable, non-toxic and importantly minimise the unnecessary immune responses of antigens while present in blood and tissue fluids.
Therefore, biomaterials and biomimetic approaches to nanomaterials can interact with virus particles and elicit immune responses with highly‑potent neutralising antibodies in a safe and effective manner. These materials are currently being extended to prevent SARS-CoV-2 infection. We conclude that further collaborative research in engineering biomaterials and nanomaterials has a high potential to overcome the challenges present in the development of next generation vaccines.
About the authors






References
- Bookstaver M, Tsai S, Bromberg J, Jewell C. Improving vaccine and immunotherapy design using biomaterials. Trends in immunology. 2018, 39 (2), 135-150.
- Yenkoidiok-Douti L, Jewell C. Integrating biomaterials and immunology to improve vaccines against infectious diseases. ACS Biomaterials Science & Engineering. 2020, 6 (2), 759-778.
- Shields IV C, Wang L, Evans M, Mitragotri S. Materials for immunotherapy. Advanced Materials. 2020, 32 (13), 1901633.
- Kumari S, Chatterjee K. Biomaterials-based formulations and surfaces to combat viral infectious diseases. APL bioengineering. 2021, 5 (1), 011503.
- Jones K. Biomaterials as vaccine adjuvants. Biotechnology Progress. 2008, 24 (4), 807-814.
- Cossette B, Kelly S, Collier J. Intranasal Subunit Vaccination Strategies Employing Nanomaterials and Biomaterials. ACS Biomaterials Science & Engineering. 2020.
- Heinrich M, Martina B, Prakash J. Nanomedicine strategies to target coronavirus. Nano Today. 2020, 100961.
- Jackman J, Yoon B, Ouyang L, Wang, N, Ferhan A, Kim J, Majima T, Cho N. Biomimetic nanomaterial strategies for virus targeting: Antiviral therapies and vaccines. Advanced Functional Materials. 2020, 2008352.
- Allahyari M, Mohit E. Peptide/protein vaccine delivery system based on PLGA particles. Human Vaccines & Immunotherapeutics. 2016, 12 (3), 806-828.
- Petrovsky N, Cooper P. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015, 33 (44), 5920-5926.
- Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017, 9 (2), 12.
- Nisini R, Poerio N, Mariotti S, De Santis F, Fraziano M. The multirole of liposomes in therapy and prevention of infectious diseases. Frontiers in immunology. 2018, 9, 155.
- McKay P, Hu K, Blakney A, Samnuan K, Brown J, Penn R, Zhou J, Bouton C, Rogers P, Polra K. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nature Communications. 2020, 11 (1), 1-7.
- Guillot A, Cordeiro A, Donnelly R, Montesinos M, Garrigues T, Melero A. Microneedle-based delivery: An overview of current applications and trends. Pharmaceutics. 2020, 12 (6), 569.
- Kim E, Erdos G, Huang S, Kenniston T, Balmert S, Carey C, Raj V, Epperly M, Klimstra W, Haagmans B. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020, 55, 102743.
- Zhang R, Billingsley M, Mitchell M. Biomaterials for vaccine-based cancer immunotherapy. Journal of Controlled Release. 2018, 292, 256-276.
Related topics
Antibodies, Biopharmaceuticals, Immunology, Nanomedicine, Proteomics, t-cells, Vaccine


