Novel RNA-targeting molecule can halt SARS-CoV-2 replication
Posted: 1 October 2020 | Hannah Balfour (Drug Target Review) | No comments yet
The C5 compound targets the frameshifting element that allows SARS-CoV-2 to effectively replicate and marks the genome for destruction to stop the infection spreading.

Researchers have created a drug-like compound which can inhibit SARS-CoV-2 replication within host cells and mark the genetic material of the virus for destruction. The drugs target the coronavirus’ frameshifting element, which the researchers liken it to a car’s clutch, that is required for changes to the reading frame of ribosomes translating proteins from the viral genome. Without the frameshifter, host cells cannot produce all the proteins required to create new copies of SARS-CoV-2.
SARS-CoV-2, the virus that causes COVID-19, and other viruses spread by releasing their genome into cells and hijacking the cellular machinery of those hosts to replicate it, producing new copies of the virus which are then released to spread the infection further. In order to achieve this, their genetic material has to be compact enough to efficiently enter cells.
In the case of SARS-CoV-2, this is accomplished by having a single positive-sense stranded RNA molecule, which – dependent on the reading frame of translating ribosomes – encodes multiple proteins needed to assemble new virus. Reading frames are a way of dividing sequences of nucleotides into non-overlapping triplets, when translating RNA into protein, each triplet encodes a single amino acid which is added onto the growing protein. The clutch-like frameshifting element can force ribosomes, the cellular machinery for translating RNA into protein, to pause, slip to a different reading frame and then restart protein assembly anew. This process enables multiple different proteins to be produced from the same RNA sequence.
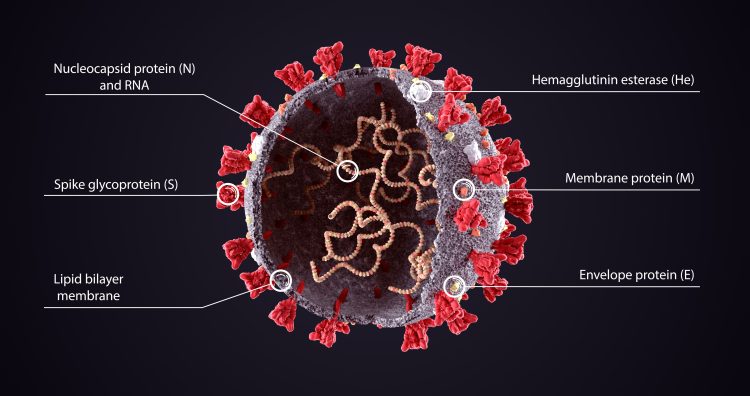

Structure of a SARS-CoV-2 viral particle: the single internal RNA strand is the genome of the virus.
While stopping this process may be desirable, it has previously been difficult to get orally administered medicines to bind to RNA. However, Scripps Research Institute chemist Dr Matthew Disney and colleagues have been developing and refining tools to do so over more than a decade.
In their study published in ACS Central Science, the team collaborated with Iowa State University Assistant Professor Dr Walter Moss to analyse and predict the structure of molecules encoded by the viral genome, in search of its vulnerabilities. They focused on the frameshifting element because it is essential for viral replication, so disrupting its function should stop the virus altogether, and because it features a stable hairpin-shaped segment. They theorised that binding a drug-like compound to this segment should disable its ability to control frameshifting.
Using a database of Disney’s RNA-binding chemical entities, they identified 26 candidate compounds that could bind to the frameshifting element. Further testing with different variants of the frameshifting structure revealed three candidates that bound them all well.
The team then experimented with the compounds in human cells carrying the SARS-CoV-2 frameshifter. Those tests revealed that C5 had the most pronounced effect, in a dose-dependent manner, and did not bind unintended RNA (ie, that of the host cell).
Disney said after these experiments they engineered the C5 compound to carry an RNA editing signal that causes the cell to specifically destroy the viral RNA. The RNA editor signals to nucleotide disposal systems found within the host cell to destroy the genome of the SARS-CoV-2 virus. His system is called RIBOTAC (Ribonuclease Targeting Chimera).
Adding a RIBOTAC to the C5 anti-SARS-CoV-2 compound increases its potency by tenfold, Disney said. However, he cautioned that this was a proof-of-concept study and a lot more research will be required to bring this drug to clinical trials, primarily because it has a completely novel mechanism of action. The next step, said Disney, is to test it against a whole SARS-CoV-2 virus.
He concluded that their study suggests other RNA viral diseases may eventually be treated through this strategy and that they hope it puts “RNA at the forefront of modern medicinal science as a drug target”.
Related topics
Cell culture, Cell-based assays, Drug Development, Drug Discovery, Drug Leads, Drug Targets, In Vitro, Research & Development, Small Molecules, Therapeutics
Related conditions
Coronavirus, Covid-19
Related organisations
Iowa State University, The Scripps Research Institute (TSRI)
Related people
Dr Matthew Disney, Dr Walter Moss


