How RNA therapeutics can be used to treat retinitis pigmentosa
Posted: 5 April 2021 | Sahm Nasseri (PYC Therapeutics) | No comments yet
Sahm Nasseri discusses promising pre-clinical results of an RNA-based therapeutic developed to treat retinitis pigmentosa type 11.

How can RNA therapeutics treat retinal diseases, including RP11?
Inherited retinal diseases (IRDs) have a genetic driver, which makes them strong candidates for treatment with RNA therapeutics. Retinitis pigmentosa type 11 (RP11) is an autosomal dominant IRD. RP11 patients typically start experiencing symptoms in early childhood with their visual function and functional vision getting progressively worse so that by age 40 they are often legally blind. There are currently no treatments available for patients with RP11.
…there have been excellent advances in identifying delivery solutions for RNA therapeutics”
RP11 is a haploinsufficiency disease, meaning that one allele of the gene in question (for RP11, the critical gene is called PRPF31) works normally while the other allele carries a mutation and does not produce a protein with normal function. In RP11, the result is insufficient protein in the retina, ultimately leading to retinal disease. For haploinsufficiency diseases such as RP11, RNA therapeutics are uniquely placed to upregulate the deficient protein through a variety of mechanisms – the upregulation of the deficient protein, often to match levels produced in healthy people, is critical to addressing the disease.
Safe and efficient delivery of the RNA therapeutic to its target tissue and cell has remained a critical challenge to overcome for RNA therapeutics. PYC Therapeutics has been able to design RNA therapeutics to target specific disease conditions, overcoming the challenge of delivering therapeutics by using our library of cell-penetrating peptides (CPPs) that we conjugate to our RNA therapeutics to enable their delivery. Our peptides are naturally-derived, meaning we can safely and effectively interact with human cells. This approach utilises precision RNA-targeted therapies enhanced by an ability to enter cells where they can act, thereby increasing the potential for a favourable outcome.
How did you develop VP-001?
VP-001 was developed by PYC’s Chief Scientific Officer, Dr Sue Fletcher, in collaboration with Dr Fred Chen, a research ophthalmologist at the Lion’s Eye Institute, Australia. Their insight was that certain patients who carry a mutated copy of the critical gene in RP11, PRPF31, have very late onset of the disease – and some never saw any symptoms emerge (so called ‘non-penetrant carriers’). This varying penetrance was reported to be driven by the level of protein expression from the remaining healthy copy of PRPF31. Fletcher and other PYC scientists, Dr Janya Grainok and Dr Ianthe Pitout, then explored drivers of this varying expression and focused on a ‘modifier gene’ called CNOT3 that could be targeted with phosphorodiamidate morpholino oligomers (PMOs) to increase the level of PRPF31 protein in patients’ retinal cells.
Can you explain the mechanism of action of VP-001?


Importantly, this mechanism of action allows us to treat patients in a mutation agnostic manner, as the drug solely looks to increase production from the healthy, normally functioning allele of the gene.
The CPP component of VP-001 is critical to enable uptake of the PMO into the critical cells affected in a patient’s retina, particularly the retinal pigment epithelium (RPE) cells.
What did your pre-clinical studies show? What were your most promising findings?
We are very excited about our pre-clinical data and look forward to continuing to develop VP-001 to bring a therapy to patients with RP11. We have three main types of pre-clinical data at this point.
First, our mouse model data show a clear dose dependent response in exon-skipping, an important marker of efficacy. Importantly, we are able to show effectiveness at much lower doses (up to 10x lower dose) than some other RNA therapeutics have shown in similar in vivo ocular models. This highlights the effectiveness of our CPP delivery platform in reaching the critical cells in the retina. Additionally, we are able to show a sustained effect more than 28 days after administration to mice. This highlights the benefit of the PMO cargo, which is highly stable and resistant to intracellular degradation. This sustained effect could translate into durable, sustained and meaningful benefits.
Second, our patient derived organoid models highlight that VP-001 is able to upregulate the target PRPF31 protein well above the level we believe is the disease correction threshold. Essentially, we are able to upregulate the PRPF31 protein to levels seen in family members who have no symptoms of RP11. Beyond upregulation of the protein, we have also seen good results with respect to improvements in functional deficits associated with RP11 in patient derived organoid models – particularly restoration of the blood-retinal barrier. See Figure 1 for a visual demonstration of how VP-001 can correct structural deficiencies in patient-derived RPE cells, that are one of the key
causes of vision loss in RP11 patients.
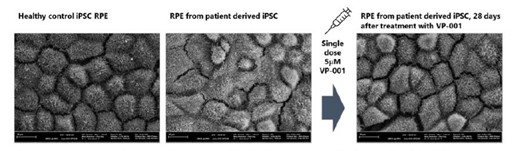

Figure 1: Scanning electron microscopy of RPE cells derived from control and patient iPSCs. Images selected as representative of full dataset.
Third, we have not observed evidence of toxicity following administration of VP-001 in our animal models. This gives us confidence that the treatment could be effective and safe at the doses required to enable correction of the disease.
What challenges did you face during your research?
We remain foundationally motivated by the urgent need to create a solution for patients suffering from RP11 – it is this inspiration that helps us overcome the challenges of taking a drug candidate from early discovery through non-clinical development into the clinic. With regard to VP-001, a key challenge was working up and optimising the patient derived models – patient induced pluripotent stem cell (iPSC)-derived retinal organoids and RPE monolayers. These models are becoming the pre-clinical gold-standard for IRDs, but they are not simple or quick models to get up and running. The process of re-differentiating patient samples to iPSCs, then differentiating to the target cell type can take well over six months.
What are the next steps of development for VP-001?
We are moving into Investigational New Drug (IND) stage enabling studies in larger animals such as rabbits and non-human primates in the second half of 2021. These studies will be important to help us understand the pharmacokinetic profile of the drug, the ocular distribution of the drug and confirm our preliminary small animal safety findings in larger animals. Our goal is to submit an IND to the US Food and Drug Administration (FDA) during the first half of 2022.
In parallel with these pre-clinical development steps, we are defining our clinical and regulatory strategies and are looking forward to engaging with the FDA on our plans in the coming months.
How do you see RNA therapeutics developing in the next five to 10 years?
Our peptides are naturally-derived, meaning we can safely and effectively interact with human cells”
RNA therapeutics hold a very important potential in addressing a wide range of diseases for which there are no or limited treatments today. Under the umbrella of genetic medicines, these are truly exciting developments that offer tremendous hope in addressing unmet needs in the future. We are excited to contribute to the progress of the exciting RNA field with the continued development of VP-001 and our other pipeline drug candidates.
While there have been excellent advances in identifying delivery solutions for RNA therapeutics, there is still great need to improve systemic delivery and delivery to specific organs such as the brain. There are a broad set of neurodegenerative diseases, many of which have a genetic origin, for which solutions are needed. We also expect to see continued innovation in the chemical modification of RNA therapeutic cargos to improve delivery, effectiveness and durability of response. With an explosion in
development of genetic medicines, including RNA therapeutics but also gene therapies, we anticipate a sharper understanding of which modality is best suited for a given therapeutic application and disease indication. Even within RNA therapeutics, there are a range of modalities and more work is required to identify precisely which modality is most applicable to a given problem. This improved understanding will ultimately help the biotech industry to design more tailored and more effective solutions to address unmet patient needs.
Sahm Nasseri holds an extensive background in commercial drug development from his roles over the past 7 years while in the USA with Merck & Co, one of the world’s leading global biopharma companies. In that time, Sahm has built experience leading product teams and commercial strategy across a range of therapeutic areas, modalities and geographies as well as within Merck’s investor relations and business development functions. Prior to Merck, Sahm was a consultant with McKinsey & Company in Sydney. He holds a Bachelor of Chemical Engineering with Honours from the University of New South Wales and an MBA from Columbia Business School in New York City.
Related topics
Biopharmaceuticals, Drug Development, Drug Leads, Gene Therapy, Genomics, In Vitro, In Vivo, Organoids, RNAs
Related conditions
Retinitis pigmentosa (RP), Retinitis pigmentosa type 11 (RP11)
Related organisations
Lion’s Eye Institute, US Food and Drug Administration (FDA)
Related people
Dr Fred Chen, Dr Ianthe Pitout, Dr Janya Grainok, Dr Sue Fletcher


