MALP cells as a potential target for osteoporosis drug development
Posted: 23 November 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Researchers have found that bone marrow adipogenic lineage precursor (MALP) cells may initiate the production of osteoclasts and drive bone remodelling in osteoporosis.
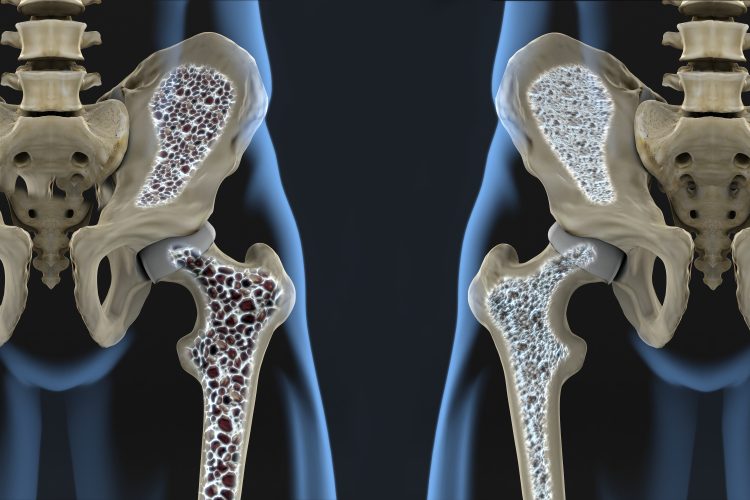

A new study suggests therapies able to target bone marrow adipogenic lineage precursor (MALP) cells and regulate bone remodelling could be effective against osteoporosis.
According to Perelman School of Medicine researchers at the University of Pennsylvania, US, osteoporosis is caused when the balance between osteoblasts, which secrete the materials necessary to form new bone, and osteoclasts, which absorb old bone material to make way for the new, becomes disrupted. They said that in osteoporosis, overactive osteoclasts remove bone faster than it can be reformed by osteoblasts, resulting in bones that are less dense and more susceptible to fracture.
The general consensus in scientists is that osteoblasts initiate the production of osteoclasts to begin the remodelling of bone, but until now the role of adipocyte lineage cells, such as MALPs, in regulating the resorption of bone was not known.
Earlier in 2020, Dr Ling Qin, an associate professor of Orthopaedic Surgery at the Perelman School of Medicine, and colleagues showed that the precursors for adipocytes, MALPs, influence bone turnover. According to the team, MALPs, not osteoblasts, have direct cell-to-cell contact with osteoclasts and they also secrete RANKL, a protein essential for forming osteoclasts, at a high level.
Working from these findings, the researchers studied mice with RANKL deficiencies in their MALPs. In their new paper, they found that after RANKL deficient mice turned one month old, they had a much higher bone density in their long bones and vertebrae (60 to 100 percent higher) than typical mice. The team classified this as “a drastic increase” in bone mass.
They also found that RANKL deficient mice had a smaller number of osteoclasts. Since they found no difference in the function or number of osteoblasts, the team concluded that MALPs and their RANKL secretions are the main driver of osteoclast function and the absorption of existing bone.
“By identifying what appears to be the full function of MALP cells, we believe that we have uncovered an extremely promising target that would never have been considered before,” Qin said. “If their RANKL secretions can be reliably disabled, it could rebalance bone remodelling in people with osteoporosis and allow for osteoblasts and osteocytes to ‘catch up.'”
She concluded: “Discovering new cellular and molecular mechanisms to control bone turnover will enable fine-tuning of existing therapies or design of novel therapeutics. For example, with the advance of gene-editing technology and novel cell-specific delivery approaches, in the future it would be possible to regulate MALP behaviour as a therapy for bone disorders like osteoporosis.”
This research was published in the Journal of Clinical Investigation.
Related topics
Disease research, Drug Targets, In Vivo, Therapeutics
Related conditions
Osteoporosis
Related organisations
The Perelman School of Medicine at the University of Pennsylvania
Related people
Dr Ling Qin


