Mini lung organoids reveal early SARS-CoV-2 infection process
Posted: 23 October 2020 | Victoria Rees (Drug Target Review) | 1 comment
Using lung organoids, researchers have shown that 48 hours after SARS-CoV-2 infection, the innate immune response began.

Mini lung organoids grown from tissue have provided a team of scientists from South Korea and the UK important insights into how COVID-19 damages the lungs. The researchers detail the mechanisms underlying SARS-CoV-2 infection and the early innate immune response in the lungs.
The researchers say that the main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be the alveoli.
The team used tissue donated to tissue banks at the Royal Papworth Hospital National Health Service (NHS) Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital, South Korea, to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organising alveolar-like three-dimensional (3D) structures that mimic the behaviour of key lung tissue.
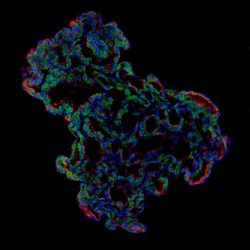

Representative image of 3D human lung alveolar organoid showing alveolar stem cell marker, HTII-280 (red) and SARS-CoV-2 entry protein, ACE2 (green) [credit: Jeonghwan Youk, Taewoo Kim and Seon Pyo Hong].
Dr Joo-Hyeon Lee, co-senior author, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in South Korea who had been diagnosed with the COVID-19 virus. Using a combination of fluorescence imaging and single cell genetic analysis, the researchers were able to study how the cells responded to infection from the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response and the cells started fighting back against infection.
The team found that sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than 10 days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants and revealing the damage processes of the virus in human alveolar cells,” said Dr Young Seok Ju, co-senior author. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
The study was published in Cell Stem Cell.
Related topics
Disease research, Organoids, Research & Development
Related conditions
Covid-19
Related organisations
Addenbrooke's Hospital, Cambridge University NHS Foundations Trust, Royal Papworth Hospital NHS Foundation Trust, Seoul National University Hospital
Related people
Dr Joo-Hyeon Lee, Dr Young Seok Ju



Nice answers in return of this query with firm arguments and explaining all
on the topic of that.