New virus surface characterisation technique developed
Posted: 9 January 2020 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have demonstrated the success of a new single-particle method of studying the surface of viruses, which could improve vaccine purification and the development of gene therapies.

A study has shown that it is possible to characterise the surface of proteins using a single-particle method. These findings could improve the possibilities for vaccine purification and making gene therapy treatments for eye diseases and muscular dystrophy.
Caryn Heldt, director of the Health Research Institute at Michigan Technological University, US, led the study into virus surface chemistry. Her work focuses on using surface charge to determine a virus’ isoelectric point, a common way to characterise viruses.
However, instead of using bulk characterisation to conduct her study, she used a single-particle method that utilises atomic force microscopy (AFM). The adhesion between the AFM probe and the sticky virus surfaces can be measured with chemical force microscopy (CFM).
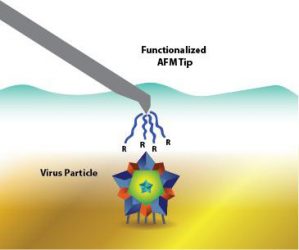

An isoelectric point is a common way to characterise viruses. However, it’s not easy. To improve manufacturing for vaccines and gene therapy, a Michigan Tech team uses surface charge to determine the isoelectric point of different viruses. Specifically, they use a single-particle method with atomic force microscopy (AFM) (credit: Jess Brassard/Michigan Tech).
“So we have these bulk methods where we put a virus in solution and we characterise the solution,” said Heldt. “But if your virus isn’t completely purified, which is also difficult to do, then your characterisation of your bulk solution means you’re characterising everything in that solution.”
Heldt explains that as a big, complex molecule, a virus reaches its isoelectric point when all its negative and positive charges balance out. At a particular pH, the virus has a neutral charge; so, if a positive charge is required then the pH can be altered to below the isoelectric point and vice versa.
Heldt’s team demonstrated that they could make the AFM probe positive or negative, then scan a solution across different pHs to determine a virus’ isoelectric point. To verify that the method worked, the team used two viruses: non-enveloped porcine parovirus (PPV), which has a well-documented isoelectric point and enveloped bovine viral diarrhea virus (BVDV), which does not have a known isoelectric point. The methods were shown to match up.
“So now we can try and predict chromatography conditions with just a small amount of virus,” Heldt said, explaining that chromatography uses surface charge to determine whether a virus is present in a medical test or for vaccine purification. “Also, we have preliminary data that shows this could be helpful for manufacturing viruses that could be modified and used to target specific genes to help with diseases like muscular dystrophy and some retinal diseases.”
For gene therapy, using many inactive virus capsids that a body’s immune system would fight is not an ideal treatment; CFM could more easily discern inactive from active capsids, which could then be purified for a more effective treatment, say the researchers.
The results were published in Langmuir.
Related topics
Drug Targets, Imaging, Microscopy, Research & Development, Targets, Vaccine
Related organisations
Health Research Institute at MTU, Michigan Technological University (MTU)
Related people
Caryn Heldt


