Monitoring the potency of cell therapies using bioelectronic assays
Posted: 24 May 2021 | Dr Jim Ross (Axion Biosystems) | No comments yet
Chimeric antigen receptor (CAR) T and natural killer (NK) therapies may become the future of cancer treatment. Dr Jim Ross explains how bioelectronic assays are a non-invasive, label-free approach built for real-time, dynamic assessment of cell therapy potency.


Personalised medicine is now within reach for thousands of cancer patients thanks to immunotherapies such as CAR T cells. This cell therapy involves training a patient’s immune system through genetic engineering to recognise tumour cells as foreign bodies and attack them directly, without attacking healthy tissue. In 2017, the US Food and Drug Administration (FDA) approved Kymriah, the first CAR T-cell therapy, for the treatment of B-cell acute lymphoblastic leukaemia. Now, patients across the US can access the treatment at any one of over 130 treatment centers.1
Immune cell potency testing will likely become a routine step in cancer therapy manufacturing”
CAR T-cell therapy’s applications continue to grow, gaining more approvals every year. One forecast2 predicts that the CAR T-cell market will reach a size of $6.1 billion by 2030. In their continued search for safer and more effective therapies, biopharmaceutical companies are now examining the therapeutic potential of another cell type – NK cells. Like CAR T cells, NK cells are cytotoxic lymphocytes and can be used as therapy because of their ability to not only target and kill cancer cells, but also enhance the T-cell and antibody response. Compared to CAR T cells, NK cells may be safer, more effective and cheaper to manufacture.3
However, with this new growth and innovation comes new challenges. Compared to traditional pharmaceutical drugs, cell therapies are not straightforward to develop and manufacture. These therapies use living cells that operate according to rules we do not yet fully understand and cannot always control. Therefore, manufacturers need to utilise assays that help identify the right molecular targets, understand critical pathways and assess manufacturing processes. The right assay can inform decisions from development to manufacturing and ensure that manufacturers can prepare the data that regulators require as CAR T cells progress through clinical trials.
Use of cell therapies
CAR T cells represent an estimated 57 percent of the cell immunotherapy space and identifying the right targets for these cells is critical to ensuring their safety and efficacy.4 Most of these cell therapies treat blood tumours by targeting CD19, a marker highly expressed in B cells. However, CD19 is not found in every cancer, meaning other targets must be discovered to treat all cancers. Solid tumours, for instance, rarely express a single selective target.5 Many antigens found on solid tumours may also be expressed at low levels in normal tissue, which could cause CAR T cells to target healthy cells and cause adverse events. Consequently, target antigens must be carefully selected to ensure the therapy effectively eliminates tumour cells while ignoring normal cells. Researchers are taking many approaches to combat these on-target/off-tumour effects, from developing therapies that target combinations of markers to improve cancer specificity and overcome antigen loss, to engineering kill switches to halt a therapy that has led to adverse events.
Identifying the right targets, however, is only the first step. Optimising transduction efficiency of the CAR gene, cell expansion protocols and cryopreservation all require a means to assess therapies in a meaningful way. One tool that can be helpful in this area by ensuring proper cell function is the in vitro potency assay. This type of assay can produce data that is key to decision-making in clinical trials and to ensuring a smoother transition to the end stage.
Ideally, this potency assay would provide the best data to inform the design of cell therapies: to test, rank and select the best candidates to move forward. It would not only reliably quantify immune cell-mediated killing at physiologically relevant cell ratios, but it would also give a complete view of a candidate’s functionality. With the wide array of potential targets, cell sources and the myriad of manufacturing processes that may influence a product, it should be conducive to screening, while simple enough to conserve cells, time and labour.


Figure 1: Interdigitated electrodes embedded in the cell culture substrate at the bottom of each well in a microtitre plate
Scientists have many tools at their disposal to measure cytotoxicity, but many common approaches are not ideal for capturing potency of CAR T cells. The chromium release assay, for example – traditionally the most commonly used method to measure immune cell cytotoxicity – is an endpoint assay that requires significant technical skill and hazardous reagents. Like other endpoint assays, such as LDH, MTT and flow cytometry, it only captures information at a single predefined time point. The maximum killing response may occur at different times across donors or immune-cell exhaustion may allow the target cancer cells to begin proliferating again and all of these important moments could be missed if observation timepoints are predefined. More specifically, a single endpoint will fail to capture the kinetics of the response and may provide misleading information if an important time point is missed.
Bioelectronic assays are emerging as a useful tool that can enable scientists to monitor the real-time interactions between immune cells and tumour cells. Embedded in the bottom of each well in a microtitre plate, bioelectrodes (Figure 1) can non-invasively detect cells, without labels, by measuring the degree to which the cells in the well happen to impede a small electrical current passing through it.6
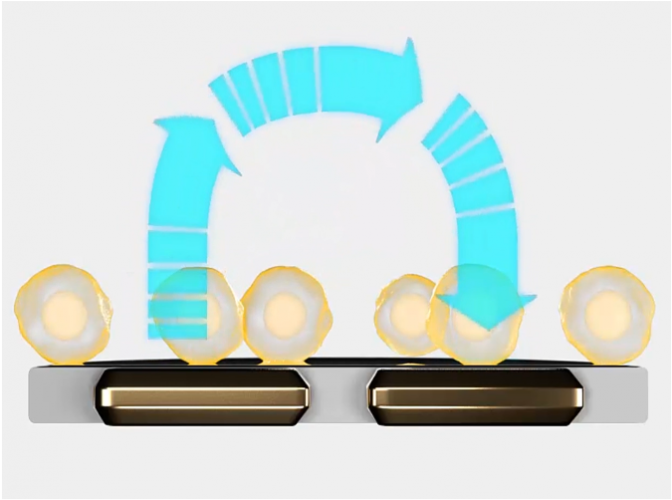

Figure 2: The electrodes detect small changes in the impedance of current flow caused by cell presence, attachment and behaviour
An impedance-based system can continuously monitor tumour cytolysis (Figure 2). As living cells attach to a well, impedance rises. As cells die or detach, impedance decreases. This enables scientists to monitor the long-term dynamics of cell death, quantifying the potency of CAR T and NK batches. Since the bioelectronic assay itself does not interact with the cell culture, the same cultures may be multiplexed to determine other quality parameters, like verifying CAR expression or target specificity (Figure 3).
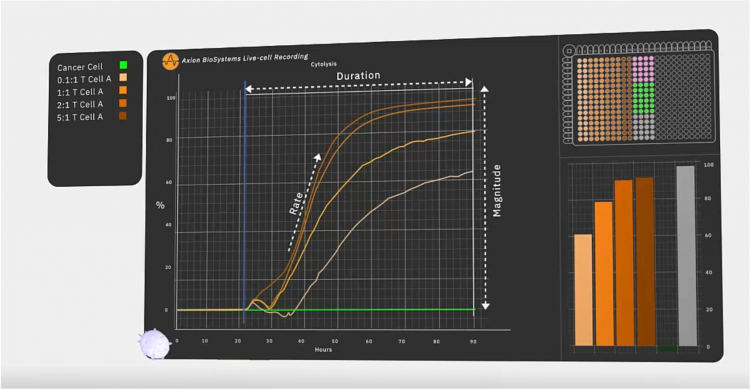

Figure 3: In an immunotherapy potency assay, the target cancer cells are added to the well first. After they attach, they grow and divide over the next 22 hours. Then, effector cells are added, such as engineered or native T cells and any additional therapeutic agent like a checkpoint inhibitor. As these cells and other agents kill the cancer cells, the platform calculates percent cytolysis automatically by comparing each well to the untreated (no effector) and fully lysed wells. In a live-cell assay, every timepoint is accessible among the continuous data. The speed at which effector cells kill target cells, how many are killed and how long the effect lasts are used to quantify the potency.
In a recent study7 to validate GD2 as a potential target, Associate Professor of Regenerative Medicine Lohitash Karumbaiah, PhD and his colleagues used one such bioelectric assay system to study the dynamics of glioma stem cell death in response to GD2-targeted CAR T cells over a seven day period. The researchers took patient-derived N08 glioma stem cells and added them to a 96-well plate outfitted with bioelectrodes and continuously monitored impedance within each well. Forty-eight hours in, the team added CAR T cells at different ratios of effector T cells to target tumour cells and continued to monitor impedance over the course of seven days. The researchers observed significant killing of at least 50 percent of target cells even at the lowest ratio of 0.1:1, suggesting that GD2 is a promising target for glioblastoma. By coupling the assay with flow cytometry, they were able to identify early markers of T-cell exhaustion, an important issue for final therapeutic efficacy.
Looking forward
Immune cell potency testing will likely become a routine step in cancer therapy manufacturing and development. The flexibility and detail of an impedance measurement offers researchers and manufacturers a new option for measuring immune cell-mediated killing during the development and testing of new CAR T and NK cell therapies. This technique might help accelerate the development pipeline of new cell-based therapies and ultimately help more people live healthier lives.
About the author
Dr Jim Ross is the Chief Technology Officer and co-founder of Axion Biosystems. He is an expert in bioelectronic assays in the life sciences, which allow scientists to precisely and continuously monitor cultured cells without labels or dyes.
References:
- Find a Kymriah treatment center [Internet]. United States: Novartis Pharmaceuticals Corporation; 2021 [updated 2021; cited 2021 Apr 29]. Available from: https://www.us.kymriah.com/treatment-center-locator/
- Research and Markets. Global CAR-T therapy market report 2020: market is expected to stabilize and reach $3,150 million in 2025 – COVID-19 impact and recovery forecast to 2030. United States: PR Newswire; 2021 [updated 2021; cited 2021 Apr 29]. Available from: https://www.prnewswire.com/news-releases…
- Natural killer cells for cancer immunotherapy: a new CAR is catching up. EBioMedicine. 2019 Jan; 39:1-2.
- Cancer Research Institute. Cancer cell therapy landscape [Internet]. United States: Cancer Research Institute; 2021 [updated 2021; cited 2021 May 11]. Available from: https://www.cancerresearch.org/scientists…
- Axion Biosystems. Technology [Internet]. United States: Axion Biosystems; 2021 [updated 2021; cited 2021 Apr 29]. Available from: https://www.axionbiosystems.com/about-us/technology
- Axion Biosystems. Video [Internet]. United States: Axion Biosystems. Available from: https://www.axionbiosystems.com/resources/video/kinetics-and-potency-gd2-car-t-cell-mediated-cytolysis-gioblastoma
Related topics
Assays, Biopharmaceuticals, Immunotherapy, Label-free, Personalised Medicine, Precision medicine, t-cells
Related conditions
Acute lymphoblastic leukaemia, Cancer, Glioblastoma, Glioma
Related organisations
US Food and Drug Administration (FDA)
Related people
Associate Professor Lohitash Karumbaiah



