Triplet immuno-oncology drug combinations – the future of treating multiple cancer
Posted: 4 June 2020 | Miguel Ferreira | No comments yet
The oncology market is saturated with new drugs that target the immune system, however, these only target part of the problem caused by cancer’s ability to hide from the immune system. Miguel Ferreira discusses why emerging three-drug combinations are poised to redefine the immuno-oncology treatment paradigm in advanced malignancies with high unmet need.

Immune system and cancer interactions
The immune system is a highly effective host-defense system which, if effectively guided, has the potential to successfully eradicate cancers. This theory was first put forth by Thomas and Burnet in 19571 and their work on immune surveillance aided William B Coley during the first application of an immuno-oncology (IO) approach in cancer treatment.2 IO drugs have redefined the treatment paradigm for every cancer type and are now a major focus of clinical research. They also have the potential to culminate in an optimal treatment approach to significantly increase cure rates among multiple cancer indications.
The IO era
Current IO treatments are derived from an initial trial-and-error strategy whereby different targets were tested to modulate an immune response. The first approach involved high doses of immune stimulatory cytokines, namely interferon and interleukin (IL)-2.3 Cytokines were considered an ideal target for IO due to their ability to promote T-cell activation, which increases the number of T-cells available to fight tumours. However, this approach was only effective in high doses since it had to saturate the tumour’s ability to suppress T-cell activation. Therefore, this strategy lost favour and, despite approval from the US Food and Drug Administration (FDA) for therapies using this approach, they were associated with high toxicities as a result of excessive immune activation, limiting their role in cancer therapy.4 Since then, pharmaceutical companies have focused on reversing immune suppression without promoting excessive immune activation. More specific approaches have been approved, namely rituximab for non-Hodgkin’s lymphoma, sipuleucel-T for prostate cancer, ipilimumab for melanoma and more recently the arrival of programmed cell death protein 1 (PD-1) inhibitors nivolumab and pembrolizumab, which are now standard IO treatments in most cancer indications.
One of many co-stimulatory pathways can then be targeted, resulting in an increase in activated T cells”
Given the limitations of monotherapies, a new age of IO therapies is emerging, whereby combination approaches are expected to provide synergistic responses that safely potentiate the immune system. Although two-drug combinations such as ipilimumab + nivolumab have been introduced and demonstrated improved treatment outcomes, they may still fall short of the overall potential of IO therapies by solely focusing on co-inhibitory receptors. Another limitation of both early and existing IO therapies is the barrier of tumours with low immunogenicity, also referred to as cold tumours. Therefore, the introduction of co-stimulatory agonists into these combinations may be the missing link in IO. To achieve this, the implemented immune checkpoint inhibitor strategy must be supplemented with the immune activation approach from the first IO treatments with immune stimulatory cytokines, but as targeted, effective and tolerable IO therapies.
Co-stimulatory and co-inhibitory receptors
T cells and cancer cells are guided by their interactions with their environment, as shown in Figure 1. They communicate using cell-surface receptors that mediate their role in the immune response. These receptors can be primarily categorised as co-inhibitory receptors or co-stimulatory receptors, aptly named for how their interaction leads to the activation or inhibition of the cell they are expressed upon. The established PD-1 inhibitors are a prime example of how targeting co-inhibitory receptors can prevent their exploitation by cancer cells for immune suppression. However, there is no established approach for co-stimulatory receptors that explores immune activation.
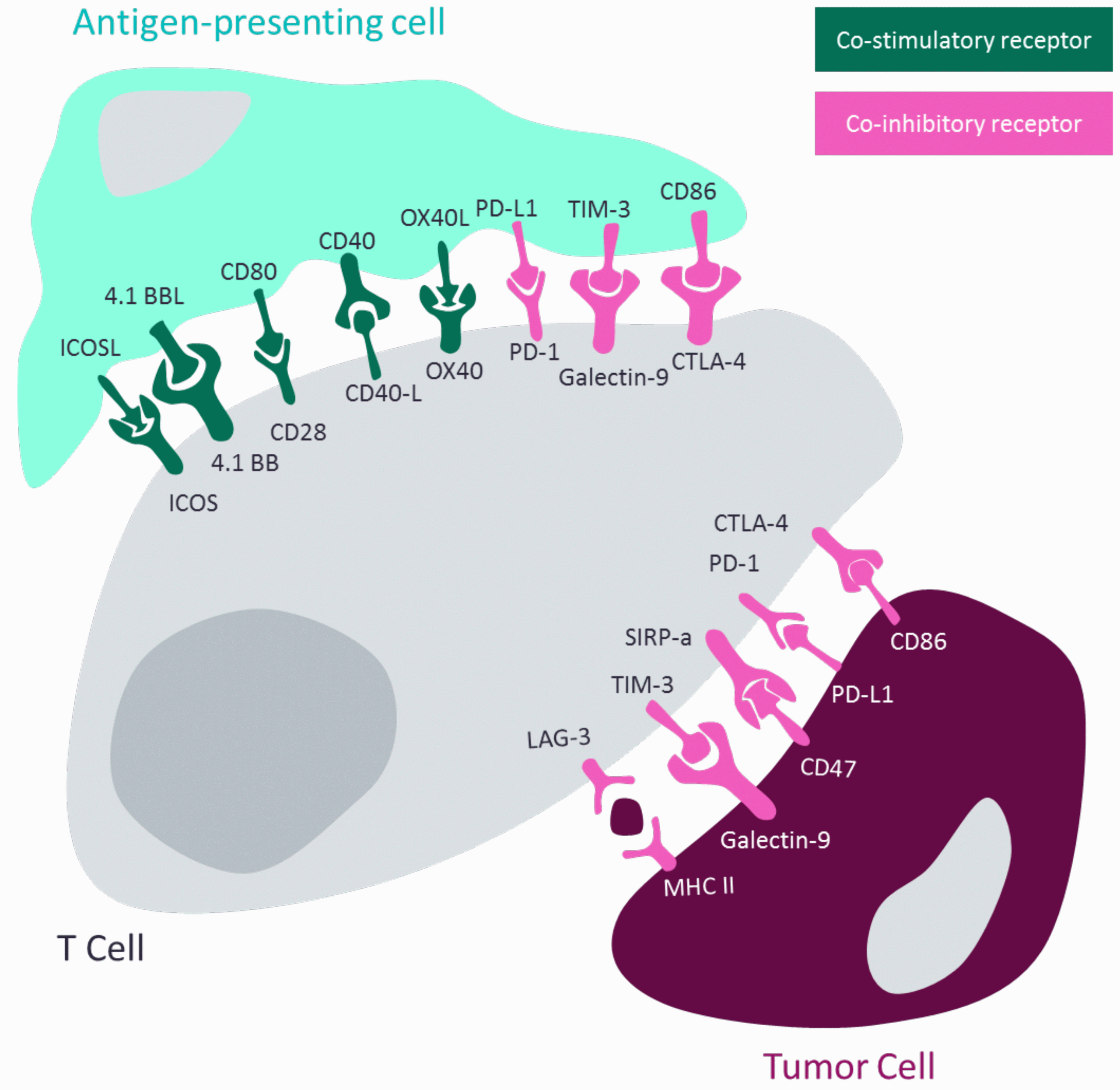

Figure 1: Diagram demonstrating how T-cell activation or inhibition is regulated by antigen-presenting cells and how tumour-cells hijack this mechanism. Adapted from Shin and Ribas 20155 and Wykes and Lewin 2017.6
The combination of a co-inhibitory component to prevent cancer cells from silencing T cells plus a co-stimulatory agonist to promote T-cell activation has the potential to be an optimal synergistic combinatorial treatment. This novel approach would result in an environment of activated T cells that would be able to target a now-exposed, treatment-sensitive tumour. The initial two-drug combination would create an ideal environment for immune response; however, a third agent could be introduced to fit the needs of specific cancers. This would vary greatly between solid and haematological malignancies. Furthermore, a triple combination has the potential to meet the unmet treatment need of cold tumours that are less likely to respond to IO approaches.
Potential co-inhibitory receptors for triplet combination therapy
The first co-inhibitory receptor-targeting drug to be approved was ipilimumab – a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor. Following the arrival of PD-1 inhibitors, CTLA-4 was a target less sought-after because it was associated with higher toxicities. Novel targets are continuously being discovered and the receptors to have currently gained interest include lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin mucin-3 (TIM-3).
LAG-3 protein is an immune checkpoint responsible for suppressing T-cell activation and cytokine release to mediate immune homeostasis.7 Currently, there are multiple pharmaceutical and biotech companies investigating anti-LAG-3 drugs, including Bristol-Myers Squibb (BMS) and Merck.8 BMS’s relatlimab has already shown promising activity according to interim results from 22 Phase II trials and the company hopes to reproduce similar results in three Phase III trials. Merck’s MK-4280 is a close second in development, although it is only involved in two Phase II trials at present.
TIM-3 is another novel immune-checkpoint inhibitor. Similar to LAG-3, it is expressed on T cells and natural-killer cells, but it is also expressed on dendritic cells and some evidence suggests that it may be expressed on macrophages.8 Unlike LAG-3, the reported ligands of TIM-3s include galectin-9 – a receptor of interest since it promotes T-cell exhaustion and the activation of suppressor cells. Galectins are a type of lectin receptor that have demonstrated behaviour as compensatory mechanisms to promote tumour-progression via tumour-associated glycosylation mechanisms. Some examples of new agents targeting galectins include BMS’s BMS-986258, Jiangsu HengRui’s SHR-1702 and Roche’s RO7121661. These are notable due to their development by companies with leading oncology portfolios and their combination trials, which include in-house PD-1/programmed death-ligand 1 (PD-L1) inhibitors.
Potential co-stimulatory receptors for triplet combination therapy
T-cell activation can be achieved directly as a result of interaction with the major histocompatibility complex (MHC)-II in antigen-presenting cells (APCs), followed by a second interaction via co-stimulatory receptors.9 This causes a non-antigen-specific activation of T cells and is the first line of defence in immune response. Alternatively, indirect T-cell activation can be achieved via B cells, which results in antigen-specific T-cell activation.10 Direct T-cell activation is currently being explored via multiple targets, but this would result in non-specific T-cell activation. While this could still promote a powerful immune response, it presents a higher risk of side effects due to the non-specificity of the approach. Some targets include the T-cell receptors CD28, OX40, 4-IBB and ICOS. Multiple pharmaceutical companies are exploring agonists towards these targets and eventual results from early trials should yield candidates for three-drug combinations.
Indirect activation of T cells via the B-cell pathway has gained interest due to its ability to create cancer-specific T cells. B-cell activation occurs when a subtype of activated T cells interacts with the B-cell CD40 receptor.11 Targeting CD40 is important as it can result in direct anti-tumour activity in B-cell lymphomas, activating innate immunosurveillance and inducing T-cell immunity.12 The direct tumour cytotoxicity is mainly relevant to B-cell malignancies, but the role of CD40 in immunosurveillance and T-cell immunity also make it an interesting candidate for general malignancies. For the latter, a B cell would require an environment where a tumour peptide is presented by an APC. In theory, this could occur after an initial non-specific T-cell response. Consequently, T cells that act on the tumour would present tumour-specific antigens to the B cell, causing the B-cells to promote the activation of T-cell specific immune responses against the tumour.
Given its potential to bridge innate and adaptive immunity and promote a potent T-cell response against the tumour, CD40 has gained a lot of interest and several drugs have been developed to that effect. Of the existing pipeline CD40 agonists, three are highlighted as having greater potential due to the strategy used to bring these drugs to market. These are Roche’s Selicrelumab, which is involved in a triple-combination trial that includes two other in-house IO drugs, Tecentriq and Avastin; Apexigen’s APX-005M, which is also involved in a triple-combination trial with BMS’s Opdivo and Cabiralizumab; and Seattle Genetics’ SEA-CD40, which is not being tested in any triplet combinations despite being an interesting drug due to BMS’s history with Seattle Genetics’ Adcetris, being explored with Opdivo.
The third component in solid tumour versus haematological malignancies
The final component of this three-drug combination can vary, depending on specific cancer types. In solid tumours, a third component may be an IO drug that targets a specific characteristic of the tumour. Roche’s triple combination trial is exploring this approach using its PD-L1 inhibitor, a novel CD40 agonist and a vascular endothelial growth factor (VEGF) inhibitor. Similarly, BMS’s trial is using a colony-stimulating factor 1 receptor (CSF-1) inhibitor as part of its triplet combination. Meanwhile, for haematological malignancies, one drug class that would suit the fluidity of this cancer type is novel bispecific T-cell engagers (BiTE). Bi-specific antibodies continue to gain popularity due to their similarity to chimeric antigen receptor (CAR)-T cell responses and off-the-shelf availability and could therefore be the missing link to an optimal IO strategy. A BiTE drug would bridge the interaction between T cells and tumour cells, behaving as an immune catalyst. Specific BiTEs would have to be developed per cancer indication depending on known biomarkers of that indication. However, BiTEs are not the only possible drug class in this strategy. Figure 2 outlines one possible scenario of the application of a triple-drug combination. The left side of the diagram shows the basis of immunosuppression with a focus on PD-1/PD-L1 interaction while the right side shows how each drug in a triplet-drug combination can theoretically result in an effective immune response. First, PD-1/PD-L1 inhibitors prevent immune suppression. One of many co-stimulatory pathways can then be targeted, resulting in an increase in activated T cells (specifically CD40-activated T cells in this example). Finally, a BiTE can be introduced to bring T cells toward a tumour-specific receptor.
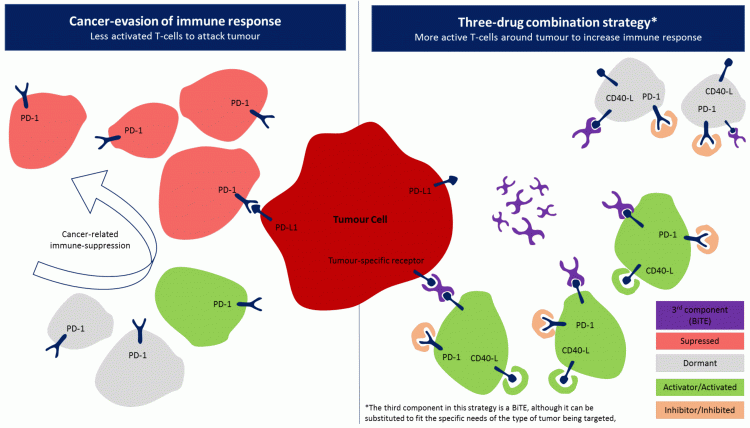

Figure 2: Example of a three-drug combination strategy using a BiTE as a third component.
About the author
Miguel Ferreira is a haematology and oncology analyst at GlobalData in London. He received a Masters’ degree in Cancer and Clinical Oncology from Barts Cancer Institute in 2018 where he completed his thesis focused on the role of aspirin in cancer prevention by attenuating cancer-promoting inflammation. Prior to this, Miguel completed his Bachelor’s degree in Biomedical Science at the University of Essex. His focus as an analyst includes creating forecast models for cancer indications and writing insight pieces mainly on the immune-oncology field.
References
- Ribatti D. The concept of immune surveillance against tumors: The first theories [Internet]. Vol. 8, Oncotarget. 2017 [cited 2020 May 1]. Available from: www.impactjournals.com/oncotarget/.
- Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Vol. 10, Frontiers in Immunology. Frontiers Media S.A.; 2019.
- Eno, MS, PA-C J. Immunotherapy Through the Years. J Adv Pract Oncol. 2017 Dec 1;8(7):747.
- Pachella, RN, MSN, AGPCNP-BC, AOCNP LA, adsen, PhD, RN, AOCNS LT, Dains, DrPH, JD, RN, FNP-CB, DPNAP JE. The Toxicity and Benefit of Various Dosing Strategies for Interleukin-2 in Metastatic Melanoma and Renal Cell Carcinoma. J Adv Pract Oncol. 2015 May 15;6(3):212.
- Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: What’s here, what’s next? Vol. 33, Current Opinion in Immunology. Elsevier Ltd; 2015. p. 23–35.
- Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Vol. 18, Nature Reviews Immunology. Nature Publishing Group; 2018. p. 91–104.
- Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, et al. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes and Cancer. 2018 May 1;9(5–6):176–189.
- Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Vol. 18, Molecular Cancer. BioMed Central Ltd.; 2019.
- Tai Y, Wang Q, Korner H, Zhang L, Wei W. Molecular mechanisms of T cells activation by dendritic cells in autoimmune diseases. Vol. 9, Frontiers in Pharmacology. Frontiers Media S.A.; 2018.
- Largeot A, Pagano G, Gonder S, Moussay E, Paggetti J. The B-Side of Cancer Immunity: The Underrated Tune. Cells. 2019 May 13;8(5):449.
- Yuen GJ, Demissie E, Pillai S. B Lymphocytes and Cancer: A Love–Hate Relationship. Vol. 2, Trends in Cancer. Cell Press; 2016. p. 747–757.
- Beatty GL, Li Y, Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Vol. 17, Expert Review of Anticancer Therapy. Taylor and Francis Ltd; 2017. p. 175–186.
The rest of this article is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related topics
Chimeric antigen receptors (CARs), Drug Development, Drug Targets, Immuno-oncology therapeutics, Immunooncology, Research & Development, t-cells, Therapeutics
Related conditions
Cancer
Related organisations
Berkeley Lights


