In vitro model could aid the development of fatty liver disease treatments
Posted: 26 January 2021 | Hannah Balfour (Drug Target Review) | No comments yet
The patient-derived model of non-alcoholic fatty liver disease (NAFLD) accurately reproduced the complex human metabolic pathways involved in the development of the disease.
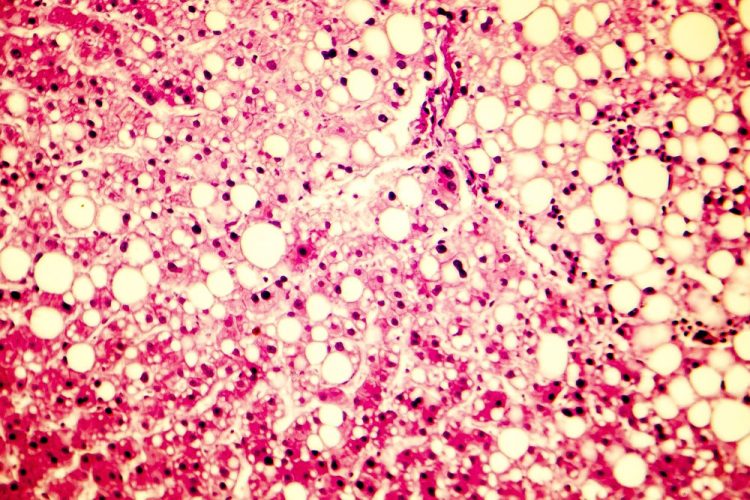

Researchers have developed a novel patient-derived in vitro model of non-alcoholic fatty liver disease (NAFLD) which could be used to screen for novel treatments. With NAFLD being widespread in the Western World, and having no approved treatments, the development of a relevant in vitro model is important.
NAFLD has four stages of development, the first is called steatosis, where lipid droplets are stored in the liver, diminishing its function – this is thought to affect 30 percent of the population. Some then develop inflammation because of the lipid droplets, progressing to the second stage called non-alcoholic steatohepatitis (NASH). Following this fibrotic scar tissue can form, the third stage, and finally liver cirrhosis can occur. Researchers have yet to fully understand the cause of this disease, despite the high number of affected individuals.
To improve their understanding of the mechanisms underlying the aetiology of NAFLD, Dr Nina Graffmann, Professor James Adjaye and the team of the Institute for Stem Cell research and Regenerative Medicine from the University Hospital Duesseldorf, Germany, differentiated induced pluripotent stem cells (iPSCs) derived from healthy donors and NAFLD patients into hepatocyte-like cells (HLCs). The HLCs were stimulated to store lipid droplets with oleic acid, mimicking the scenario of an individual consuming excess fat in their diet. According to the team, the lipid droplets produced in HLCs are akin to lipid droplets seen in the livers of NAFLD patients.
In their study, published in Biology Open, the researchers reported that the cell lines were highly heterogeneous in terms of the genes expressed and lipid droplet morphology. The scientists suggest this is due to the plethora of metabolic networks involved in the development of the disease and said it reflects the multifaceted, patient-dependent phenotypes which make NAFLD a highly complex disease. Nonetheless, the scientists were able to identify gene expression patterns that correlate with disease severity.
They also tested whether AdipoRon, a synthetic analogue of adiponectin (a molecule synthesised by human fat cells) which has been shown to positively influence hepatocyte metabolism, could reverse the steatotic phenotype of the HLCs.
The cell lines had varied responses to the treatment, but AdipoRon was shown to have a general influence on metabolism, transport, immune system, cell stress and signalling.
Dr Graffmann commented: “We could recapitulate important aspects of NAFLD with our stem cell-based culture model. We are going to use it for further studies, because established animal models cannot reproduce the complex human metabolic pathways involved in the development of the disease.”
Adjaye added: “Once again, we have shown that although iPSC- derived hepatocyte-like cells are immature in nature, ie, fetal, these cells still have immense usefulness in their application in drug discovery and for dissecting disease mechanisms such as NAFLD.”
Related topics
Cell culture, Cell-based assays, Disease research, Drug Development, Genomics, In Vitro, Therapeutics
Related conditions
non-alcoholic fatty liver disease (NAFLD), Non-alcoholic steatohepatitis (NASH)
Related organisations
University Hospital Duesseldorf
Related people
Dr Nina Graffmann, Professor James Adjaye


