Novel class of targeted cancer therapies could treat myeloid leukaemias
Posted: 16 December 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Cancer researchers have created a new class of drugs to selectively target and destroy myeloid leukaemia cells with TET gene mutations.
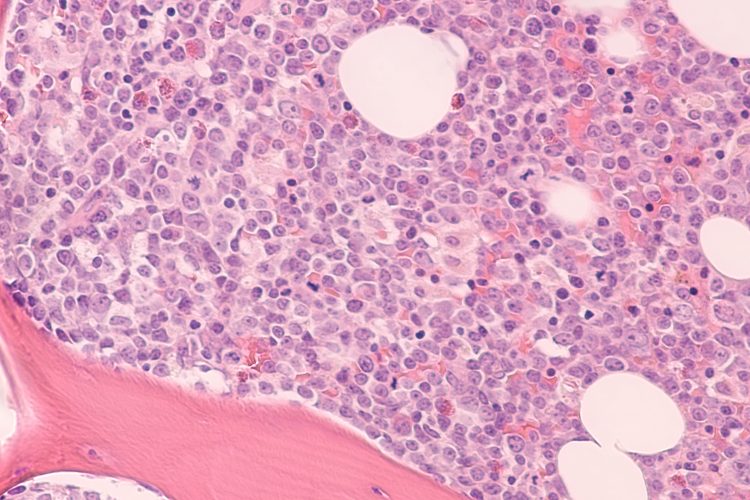

Photomicrograph of bone marrow biopsy showing myeloblasts of acute myeloid leukemia (AML), a cancer of white blood cells.
Researchers have developed a novel class of targeted cancer drug that may be highly effective for the treatment of myeloid leukaemias. According to the team, their synthetic molecule, called TETi76, was able to selectively kill cells with TET2 gene mutations, one of the most common driver mutations in myeloid leukaemias.
Myeloid leukaemias are cancers derived from stem and progenitor cells in the bone marrow that give rise to all normal blood cells. These malignancies are normally treated with chemotherapy, either alone or in combination with targeted drugs; however, the significant side-effects associated with this treatment mean a more selective/targeted treatment is desirable.
In a new study published in Blood Cancer Discovery, researchers from the Cleveland Clinic’s Taussig Cancer Institute and Lerner Research Institute, both US, describe a new pharmacological strategy to preferentially target and eliminate leukaemia cells with TET2 mutations.
“In pre-clinical models, we found that a synthetic molecule called TETi76 was able to target and kill the mutant cancer cells both in the early phases of disease – what we call clonal haematopoiesis of indeterminate potential, or CHIP – and in fully developed TET2 mutant myeloid leukaemia,” said Dr Jaroslaw Maciejewski, a practicing haematologist and chair of the Cleveland Clinic Department of Translational Hematology & Oncology Research, who has been investigating the TET2 gene for the last decade.
TET genes encode DNA dioxygenase enzymes, which remove chemical groups from DNA molecules. Their activity ultimately changes what genes are expressed and can contribute to the development and spread of disease.
TET genes act as tumour suppressors, so loss-of-function mutations are common in haematological cancers, like leukaemias. While all members of the TET family are dioxygenases, TET2 is the most powerful. Genetic TET2 deficiency has been shown to skew differentiation of blood cells and clonal expansion of progenitor and stem cells. However, its related genes – TET1 and TET3 – provide residual enzymatic activity, sufficient to facilitate the survival of these progenitor cells harbouring cancerous mutations, thereby promoting the spread of the cancer, even when TET2 is inactive.
In their study, the research team designed TETi76 to replicate and amplify the effects of a natural molecule called 2-hydroxyglutarate (2HG), which inhibits the enzymatic activity of TET genes. They hoped to selectively eliminate TET2 mutant leukaemia cells centres by targeting their reliance on this residual DNA dioxygenase activity.
“We took lessons from the natural biological capabilities of 2HG,” explained Dr Babal Kant Jha, Maciejewski’s collaborator from the Department of Translational Hematology & Oncology Research. “We studied the molecule and rationally designed a novel small molecule, synthesised by our chemistry group headed by Dr James Phillips. Together, we generated TETi76 – a similar, but more potent version capable of inhibiting not just TET2, but also the remaining disease-driving enzymatic activity of TET1 and TET3.”
The researchers studied TETi76’s effects in both pre-clinical disease and xenograft models (where human cancer cells are implanted into pre-clinical models). In both models, treatment with the novel TET inhibitor suppressed the clonal evolution of TET2 mutant cells.
While the team cautioned that additional studies would be critical to investigate the small molecule’s cancer-fighting capabilities in patients, Dr Jha said “we are optimistic about our results, which show not just that TETi76 preferentially restricts the growth and spread of cells with TET2 mutations, but also gives survival advantage to normal stem and progenitor cells.”
Related topics
Drug Development, Drug Leads, Drug Targets, Genomics, In Vivo, Oncology, Therapeutics
Related conditions
Leukaemia
Related organisations
Cleveland Clinic's Lerner Research Institute, Cleveland Clinic's Taussig Cancer Institute
Related people
Dr Babal Kant Jha, Dr Jaroslaw Maciejewski


