Roche provides update on phase III study of onartuzumab in people with specific type of lung cancer
Posted: 3 March 2014 | Roche | No comments yet
Roche announced that an independent data monitoring committee has recommended that the phase III METLung study be stopped due to a lack of clinically meaningful efficacy…
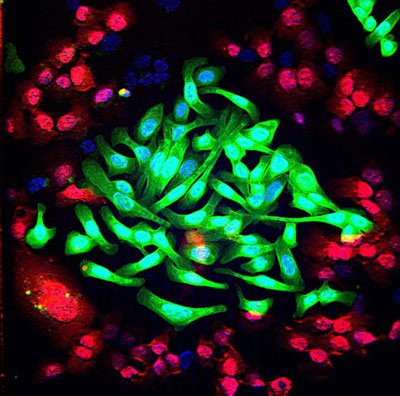

Lung cancer cells (green) are cultured together with normal lung cells (red). The triple-antibody combination EGFR, HER2 and HER3 strongly impairs the survival of tumor cells while sparing normal cells. Modified confocal microscopy image: Maicol Mancini, lab of Professor Yosef Yarden. CREDIT: Weizmann Institute of Science.
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that an independent data monitoring committee has recommended that the phase III METLung study be stopped due to a lack of clinically meaningful efficacy.
The study evaluated if onartuzumab (MetMab) in combination with Tarceva (erlotinib) helped patients with previously treated, advanced non-small cell lung cancer (NSCLC) whose tumours were identified as MET-positive live longer compared to Tarceva alone. Overall adverse event rates were generally similar between the two groups. Data will be submitted for presentation at a forthcoming medical meeting.
“These results are disappointing because new options are needed for patients with lung cancer, the most common and deadly cancer worldwide,” said Sandra Horning, M.D., Chief Medical Officer and Head of Global Product Development. “We remain committed to helping patients with lung cancer and are studying several investigational medicines in this disease.”
Roche is evaluating the implications of the METLung study results across the ongoing onartuzumab clinical programme.
About the METLung Study (NCT01456325)
- METLung is a Phase III, randomised, double-blind study evaluating the efficacy and safety profile of onartuzumab in combination with Tarceva in patients with previously treated (second- or third-line) advanced NSCLC identified to be MET-positive. The METLung study included a companion diagnostic immunohistochemistry (IHC) test that was co-developed with Ventana Medical Systems, Inc., a member of the Roche Group.
- Four hundred and ninety-nine patients were randomized to receive 150 mg of Tarceva taken daily plus either:
– Intravenous 15 mg/kg of onartuzumab every three weeks
– Intravenous placebo every three weeks - The primary endpoint is overall survival. Secondary endpoints include progression-free survival, objective response rate and safety profile.
- The results announced today are from a pre-specified interim analysis.
Related organisations
Roche
Related people
Sandra Horning


