Artificial binding protein could be developed into a novel anti-viral or cancer therapy
Posted: 18 March 2020 | Hannah Balfour (Drug Target Review) | No comments yet
Scientists have created an artificial protein able to recognise and bind cell surface carbohydrates with high affinity and selectivity.

Researchers exploring how artificial binding proteins could be used for therapeutic applications have created one which could target carbohydrates that play a role in cancer and infectious diseases.
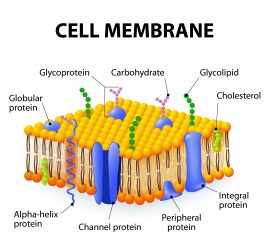

Diagram of the human/eukaryotic cell membrane: carbohydrates on the surface of human cells are bound to transmembrane proteins forming a structure called a ‘glycoprotein’.
“The recognition of specific sugar molecules, or so-called carbohydrates, is of vital importance in many biological processes,” explained Arne Skerra, laboratory director and Professor of Biological Chemistry at the Technical University of Munich, Germany.
Both pathogens and human cells have sugar structures outside of their cell membranes which allow the body to recognise them. However, proteins able to bind or recognise these carbohydrates typically have low affinity because water molecules look similar to sugar molecules and so the carbohydrates are camouflaged in the aqueous extracellular environment. Skerra and colleagues set out to change this – the artificial binding protein they created is detailed in ChemBioChem.
A novel amino acid substitute
There are 20 natural amino acids incorporated into the proteins of all living organisms.
“Using the possibilities opened up by synthetic biology, we have employed an additional artificial amino acid,” stated researcher Carina Sommer. “We have succeeded in incorporating a boric acid group, which exerts intrinsic affinity to sugar molecules, into the amino acid chain of a protein. In doing this, we have created an entirely new class of binding protein for sugar molecules.”
According to the team, this artificial sugar-binding function is superior to natural sugar binding proteins, called lectins, both in strength and specificity.
Professor Skerra explained that boron has low toxicity to humans and is therefore ideal for use in therapeutics, “but so far has largely remained unexplored.”
Scientist Dr Andreas Eichinger revealed that the team used X-ray crystallography to validate the concept of using boron to bind to sugars; the crystal structure of a model complex of the artificial protein with sugar was analysed.
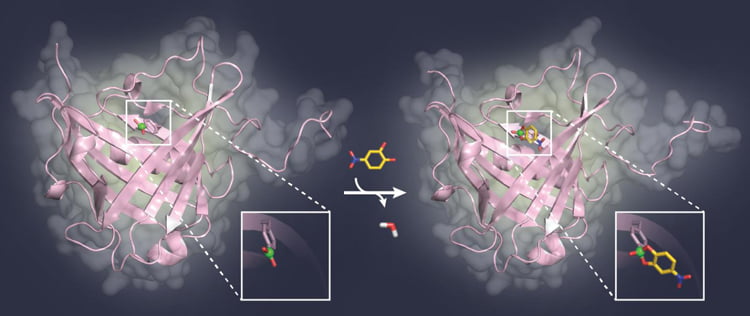

Professor Skerra’s current research findings are paving the way for the development of new types of binding proteins for biological sugar structures, which play a significant role in cancer as well as infectious diseases. – What you can see here: A model sugar ligand (yellow) binds to the boric acid group (green) in the pocket of a binding protein (pink) [credit: TUM-Chair of Biological Chemistry].
Moving into medical application
The team hope that their research could now be translated into clinical therapeutics. Professor Skerra said: “Our results should not only be used to support the future development of new carbohydrate ligands in biological chemistry, but should also pave the way for creating high-affinity agents for controlling or blocking medically-relevant sugar structures on cell surfaces.”
According to the team, these blocking agents could be useful against conditions such as cancer and infectious disease; for example, to block viruses binding to the cell surface carbohydrates to enter and infect cells. Blocking these sugar-binding functions and slowing disease progression could grant the immune system sufficient time to combat them.
Related topics
Amino acids, Drug Development, Drug Targets, Imaging, Ligands, Molecular Targets, Oncology, Research & Development, X-ray crystallography
Related conditions
Cancer, viral infections
Related organisations
Technical University of Munich (TUM)
Related people
Carina Sommer, Dr Andreas Eichinger, Professor Arne Skerra



