Remdesivir demonstrates success against MERS in rhesus macaques
Posted: 14 February 2020 | Victoria Rees (Drug Target Review) | No comments yet
The experimental remdesivir drug has shown efficacy in combatting the MERS virus in rhesus macaques, according to a new US study.


An experimental antiviral vaccine called remdesivir has successfully prevented disease in rhesus macaques infected with Middle East respiratory syndrome coronavirus (MERS-CoV), according to a new study from US National Institutes of Health (NIH) scientists. Remdesivir prevented the condition when administered before infection and improved the macaques’ symptoms when given after the animals already were infected.
Remdesivir has previously protected animals against a variety of viruses in lab experiments. The drug has been shown in pre-clinical trials to effectively treat monkeys infected with Nipah virus and Ebola, as well as being investigated as an Ebola treatment in people.
The current study involved three groups of animals: those treated with remdesivir 24 hours before infection with MERS-CoV; those treated 12 hours after infection (close to the peak time for MERS-CoV replication in these animals); and untreated control animals.
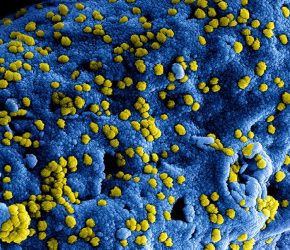

Colorised scanning electron micrograph of Middle East Respiratory Syndrome virus particles attached to the surface of an infected cell (credit: NIAID-Frederick).
The scientists observed the animals for six days and all control animals showed signs of respiratory disease. Animals treated before infection fared well: no signs of respiratory disease, significantly lower levels of virus replication in the lungs compared to control animals and no lung damage. Those treated after infection fared significantly better than the control animals. Their disease was less severe, their lungs had lower levels of virus and the damage to the lungs was less severe than in the controls.
The scientists indicate that the promising study results support additional clinical trials of remdesivir for MERS-CoV and COVID-19, the disease that SARS-CoV-2 causes. Several clinical trials of remdesivir for COVID-19 are under way in China and other patients with COVID-19 have received the drug under a compassionate use protocol.
The report from the NIH appears in the Proceedings of the National Academy of Sciences.
Related topics
Drug Development, Research & Development, Targets, Therapeutics, Vaccine
Related conditions
Covid-19, Middle East Respiratory Syndrome (MERS)
Related organisations
US National Institutes of Health (NIH)


