Cellular barcoding helps scientists understand the behaviour of stem cells
Posted: 4 January 2018 | Dr Zara Kassam (Drug Target Review) | No comments yet
Use of labelling technology in mice leads to discoveries in process of natural blood formation…

By tagging bone marrow cells of mice with a genetic label, or barcode, researchers were able to track and describe the family tree of individual blood cells as they form in their natural environment. The scientists discovered that these cells regenerate differently than their counterparts do after a blood cell transplant.
In the study, researchers tagged cells using a transposon, a piece of genetic code that can jump to a random point in DNA when exposed to an enzyme called transposase, to track blood progenitors and adult stem cells during the natural, unperturbed process of blood regeneration.
The research provides evidence for a substantially revised roadmap for normal blood regeneration or blood production in the natural environment and highlights how in those conditions blood stem cells and progenitor cells manifest unique properties.
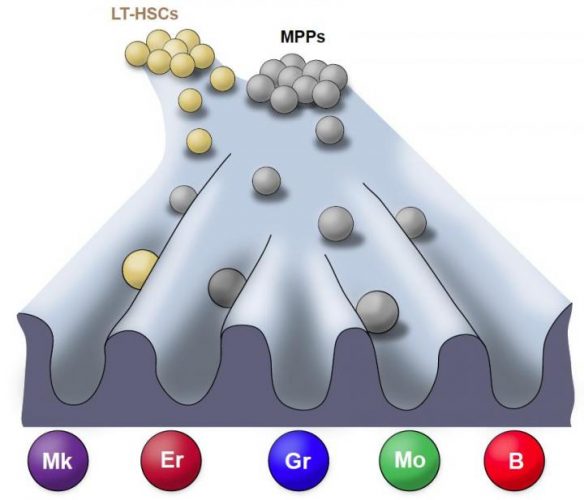

Landscape of the lineage fate (natural environment) of unperturbed haematopoiesis (the process of mature blood and immune cell production). Courtesy of the Stem Cell Program, Boston Children’s Hospital.
“The findings of this research, if applicable to humans, will have implications for blood cell transplantation, and for clinical and research methods using blood cells, such as gene therapy or gene editing,” said Dr John W. Thomas, Stem Cell and Cell-based Therapy Coordinator at National Heart, Lung and Blood Institute (NHLBI).
This study moves research a step further towards the development of blood regeneration therapies, but the researchers believe it is also applicable to a variety of cells and will yield insights about regenerating diseased or damaged tissues.
“Our results show that stem cells and their less pluripotent descendants, blood progenitors, behave somewhat differently when studied without removing them from their native environment versus when studied in a laboratory or in transplantation; leading to differences in the type of blood lineages they make,” said study’s first author Dr Alejo Rodriguez Fraticelli, from Harvard Stem Cell Institute, at the Boston Children’s Hospital.
Due to the lack of appropriate tools to study how blood forms in the natural environment of the body, the majority of studies about where individual blood cells come from have been done after a transplant. In that context, the transplanted cells would have been “perturbed,” or removed from their natural environment. According to the researchers, the current models are more likely to represent a roadmap of lineage potential for the blood cells’ natural offspring.
For Dr Fraticelli, this highlights the importance of studying blood regeneration in its native context. “Moving forward, we need to come up with methods to better predict what types of cells will be the most optimal for therapy, for instance in reprogramming cells, and editing,” he said.
The study has been published in Nature and funded by the National Heart, Lung and Blood Institute (NHLBI), part of the National Institutes of Health.
Related topics
Stem Cells
Related conditions
Blood cell transplant
Related organisations
National Institutes of Health


