Stem cell approach offers a first step in treating hypopituitarism
Posted: 14 June 2016 | Victoria White, Digital Content Producer | No comments yet
Stem cell generated functional pituitary tissue promoted normal hormone release when transplanted into rats with hypopituitarism…
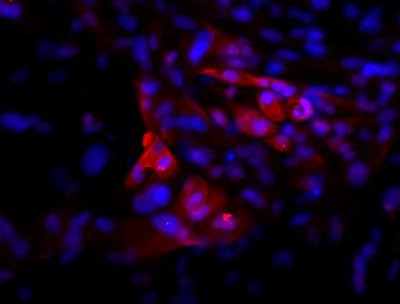

Immunofluorescence image of hPSC-derived pituitary cells after 30 days of differentiation. The cells were stained for ACTH (red) and DNA/nucleus (blue). Cells similar to the ones shown in the picture were used for the transplantation studies. CREDIT: Bastian Zimmer, Sloan Kettering Institute
Researchers have successfully used human stem cells to generate functional pituitary tissue that secretes hormones important for the body’s stress response as well as for its growth and reproductive functions.
When transplanted into rats with hypopituitarism, the lab-grown pituitary cells promoted normal hormone release. The study lays the foundation for future preclinical work.
“The current treatment options for patients suffering from hypopituitarism, a dysfunction of the pituitary gland, are far from optimal,” says Bastian Zimmer of the Sloan Kettering Institute for Cancer Research. “Cell replacement could offer a more permanent therapeutic option with pluripotent stem cell-derived hormone-producing cells that functionally integrate and respond to positive and negative feedback from the body. Achieving such a long-term goal may lead to a potential cure, not only a treatment, for those patients.”
Currently, patients with hypopituitarism must take expensive, lifelong hormone replacement therapies that poorly mimic the body’s complex patterns of hormone secretion that fluctuates with circadian rhythms and responds to feedback from other organs. By contrast, cell replacement therapies hold promise for permanently restoring natural patterns of hormone secretion while avoiding the need for costly, lifelong treatments.
Recently, scientists developed a procedure for generating pituitary cells from human pluripotent stem cells using organoid cultures that mimic the 3D organisation of the developing pituitary gland. However, this approach is inefficient and complicated, relies on ill-defined cellular signals, lacks reproducibility, and is not scalable or suitable for clinical-grade cell manufacturing.
A simple, efficient, and robust stem cell-based strategy
To address these limitations, Zimmer and Lorenz Studer of the Sloan Kettering Institute for Cancer Research developed a simple, efficient, and robust stem cell-based strategy for reliably producing a large number of diverse, functional pituitary cell types suitable for therapeutic use. Instead of mimicking the complex 3D organisation of the developing pituitary gland, this approach relies on the precisely timed exposure of human pluripotent stem cells to a few specific cellular signals that are known to play an important role during embryonic development.
Exposure to these proteins triggered the stem cells to turn into different types of functional pituitary cells that released hormones important for bone and tissue growth (i.e., growth hormone), the stress response (i.e., adrenocorticotropic hormone), and reproductive functions (i.e., prolactin, follicle-stimulating hormone, and luteinizing hormone). Moreover, these stem cell-derived cells released different amounts of hormone in response to known feedback signals generated by other organs in the body.
To test the therapeutic potential of this approach, the researchers transplanted the stem cell-derived pituitary cells under the skin of rats whose pituitary gland had been surgical removed. The cell grafts not only secreted adrenocorticotropic hormone, prolactin, and follicle-stimulating hormone, but they also triggered appropriate hormonal responses in the kidneys.
Tailoring specific cell types
The researchers were also able to control the relative composition of different hormonal cell types simply by exposing human pluripotent stem cells to different ratios of two proteins: fibroblast growth factor 8 and bone morphogenetic protein 2. This finding suggests their approach could be tailored to generate specific cell types for patients with different types of hypopituitarism. “For the broad application of stem cell-derived pituitary cells in the future, cell replacement therapy may need to be customised to the specific needs of a given patient population,” Zimmer says.
In future studies, the researchers plan to further improve the protocol to generate pure populations of various hormone-releasing cell types, enabling the production of grafts that are tailored to the needs of individual patients. They will also test this approach on more clinically relevant animal models that have pituitary damage caused by radiation therapy and receive grafts in or near the pituitary gland rather than under the skin.
Related topics
Stem Cells
Related conditions
Hypopituitarism
Related organisations
Memorial Sloan Kettering Cancer Center


