Zika virus structure revealed by Purdue scientists
Posted: 1 April 2016 | Victoria White | No comments yet
Researchers have determined the structure of the Zika virus, revealing insights critical to the development of effective antiviral treatments and vaccines…
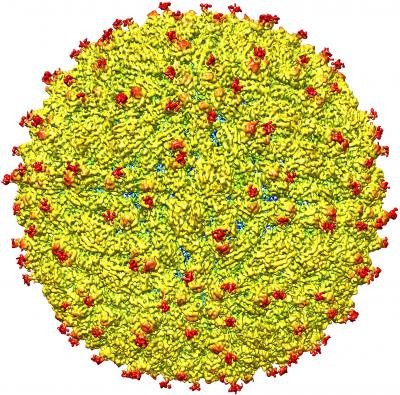

A representation of the surface of the Zika virus is shown. CREDIT: Purdue University image/courtesy of Kuhn and Rossmann research groups
Researchers have determined the structure of the Zika virus, revealing insights critical to the development of effective antiviral treatments and vaccines.
The team also identified regions within the Zika virus structure where it differs from other flaviviruses.
Any regions within the virus structure unique to Zika have the potential to explain differences in how a virus is transmitted and how it manifests as a disease, said Richard Kuhn, director of the Purdue Institute for Inflammation, Immunology and Infectious Diseases (PI4D) who led the research team with Michael Rossmann, Purdue‘s Hanley Distinguished Professor of Biological Sciences.
“The structure of the virus provides a map that shows potential regions of the virus that could be targeted by a therapeutic treatment, used to create an effective vaccine or to improve our ability to diagnose and distinguish Zika infection from that of other related viruses,” said Kuhn. “Determining the structure greatly advances our understanding of Zika – a virus about which little is known. It illuminates the most promising areas for further testing and research to combat infection.”
The Zika virus, a mosquito-borne disease, has recently been associated with a birth defect called microcephaly that causes brain damage and an abnormally small head in babies born to mothers infected during pregnancy. It also has been associated with the autoimmune disease Guillain-Barré syndrome, which can lead to temporary paralysis. In the majority of infected individuals symptoms are mild and include fever, skin rashes and flu-like illness.
Purdue University’s recently announced $250 million investment in the life sciences funded the purchase of advanced equipment that allowed the team to do in a couple of months what otherwise would have taken years, Rossmann said.
“We were able to determine through cryo-electron microscopy the virus structure at a resolution that previously would only have been possible through X-ray crystallography,” he said. “Since the 1950s X-ray crystallography has been the standard method for determining the structure of viruses, but it requires a relatively large amount of virus, which isn’t always available; it can be very difficult to do, especially for viruses like Zika that have a lipid membrane and don’t organize accurately in a crystal; and it takes a long time. Now we can do it through electron microscopy and view the virus in a more native state. This was unthinkable only a few years ago.”
The team studied a strain of Zika virus isolated from a patient infected during the French Polynesia epidemic and determined the structure to 3.8Å. At this near-atomic resolution key features of the virus structure can be seen and groups of atoms that form specific chemical entities, such as those that represent one of 20 naturally occurring amino acids, can be recognized, Rossmann said.
The team found the structure to be very similar to that of other flaviviruses with an RNA genome surrounded by a lipid, or fatty, membrane inside an icosahedral protein shell.
Zika virus has subtle structural differences to other flaviviruses
The strong similarity with other flaviviruses was not surprising and is perhaps reassuring in terms of vaccine development already underway, but the subtle structural differences are possibly key, Sirohi said.
“Most viruses don’t invade the nervous system or the developing foetus due to blood-brain and placental barriers, but the association with improper brain development in foetuses suggest Zika does,” Sirohi said. “It is not clear how Zika gains access to these cells and infects them, but these areas of structural difference may be involved. These unique areas may be crucial and warrant further investigation.”
The team found that all of the known flavivirus structures differ in the amino acids that surround a glycosylation site in the virus shell. The shell is made up of 180 copies of two different proteins. These, like all proteins, are long chains of amino acids folded into particular structures to create a protein molecule, Rossmann said.
The glycosylation site where Zika virus differs from other flaviviruses protrudes from the surface of the virus. A carbohydrate molecule consisting of various sugars is attached to the viral protein surface at this site. In many other viruses it has been shown that as the virus projects a glycosylation site outward, an attachment receptor molecule on the surface of a human cell recognises the sugars and binds to them, Kuhn said. The glycosylation site and surrounding residues on Zika virus may also be involved in attachment to human cells, and the differences in the amino acids between different flaviviruses could signify differences in the kinds of molecules to which the virus can attach and the different human cells it can infect, Rossmann said.
“If this site functions as it does in dengue and is involved in attachment to human cells, it could be a good spot to target an antiviral compound,” Rossmann said. “If this is the case, perhaps an inhibitor could be designed to block this function and keep the virus from attaching to and infecting human cells.”
The team plans to pursue further testing to evaluate the different regions as targets for treatment and to develop potential therapeutic molecules.
The team have produced an animation of Zika’s structure:
Related topics
Amino acids, X-ray crystallography
Related conditions
Zika virus
Related organisations
Purdue University
Related people
Michael Rossmann



