3D printing: A new era for personalised medicines?
Posted: 3 December 2015 | Dr Simon Gaisford (University College London School of Pharmacy) | No comments yet
3D printing (3DP) is attracting increasing interest as a new method of fabricating pharmaceutical products, especially with the recent United States Food and Drug Administration (FDA) approval of the first three-dimensional (3D) printed tablet Spritam® (levetiracetam). 3DP is considered to be an additive manufacturing technique, because regardless of their principles of action, all 3D printers create objects layer by layer on a build plate. Additive manufacturing technology has a number of advantages compared with large-scale processes (such as powder compaction) including the ability to locate the active drug at specific locations in the product, to vary size and shape, to incorporate layers with different release properties or containing different actives and, possibly most importantly, to fabricate as few as a single dosage form. If developed correctly, 3DP technology thus offers the potential to herald a new era of personalised medication, where doses or dose combinations can be tailored to the specific requirements of the patient. In this article, some of the latest types of 3D printing technology will be discussed, and their potential to pharmaceutical formulation highlighted…


3D printing technology
Printing a pharmaceutical product requires the design to be created with a computer-aided-design (CAD) software package; it must then be digitally sliced in order for the printer to fabricate it. The most widely used file format for encoding the individual layers is stereolithographic. Once encoded, the file is transferred to the printer’s driving software so the printer can fabricate the object.
The first 3DP technology was essentially a form of ink-jet printing, wherein a liquid adhesive is jetted onto a powder bed to create each solid layer. After forming each layer, a new powder bed is laid on top of the build plate and the process repeats. Once the printing is finished, excess powder is blown away and the object may be further strengthened by heat treatment. This approach is used to manufacture Spritam® tablets (Zipdose® technology) and one of the main advantages it confers on the product is rapid disintegration in the mouth (since the powder particles are only loosely bound). Powder-bed printing also offers the familiarity of using powders and so there is potential for reformulating current tablets made from compressed powders. One limitation is that it cannot be used to make hollow objects, since unbound powder will be trapped in any cavities during fabrication, which may limit the shapes of unit doses that can be manufactured.
More recently, fused-filament fabrication (FFF) – also known as fused deposition modelling (FDM) – 3D printers have become available. Their increasing popularity is down to their low cost and simplicity, which lends itself to home operation, and numerous desktop systems are available. The basic principle of operation is simple. The feedstock, which is a filament of extruded polymer, is fed through a heated tip. The polymer softens and is deposited on a build plate, which is kept cold enough to solidify the polymer. The tip is moved in a raster pattern across the build plate to create a layer of the object being fabricated. The printer then deposits polymer in the same fashion layer by layer until the final object is created. Although the commercially-available feedstock polymers are usually hard and non-ingestible (such as polyvinyl alcohol or acrylonitrile butadiene styrene), it is possible to extrude pharmaceutical grade polymers with suitable melting and solidifying temperatures for printing, and so the number of applications of FDM printing is increasing. From a pharmaceutical perspective, preparation of polymer filaments is convenient because it can be achieved with hot-melt extrusion (HME), and so the infrastructure and understanding of polymer properties exists. Furthermore, it is relatively easy to incorporate actives into the polymer filaments during extrusion.
FDM printers can easily fabricate dosage forms, such as tablets and caplets. Dosage forms will comprise individual strands that adhere together (see Figure 1) and the diameter of the strands will be approximately the size of the print tip. It takes ca. five-to-10 minutes to print each unit, although this is partly because the printers used are designed to print high-resolution objects, and so the printer tip is typically 30-100 microns in diameter. Larger diameter tips would reduce the printing time. The first report of an FDM printer being used to fabricate tablets was in 20141 and since then the number and complexity of dosage forms being printed has increased. One immediate benefit of FDM printing is that the density of the object is easily varied by altering the ‘infill percentage’ (essentially, how much polymer is deposited inside the printed object), and density is usually found to affect the rate of dissolution of drug. Another advantage is the ease with which objects with complex or unusual geometries can be printed, which would be impossible to achieve with powder compaction2. While tablets of unusual shapes might not ultimately make commercial production, they do allow exploration and validation of dissolution kinetics models.
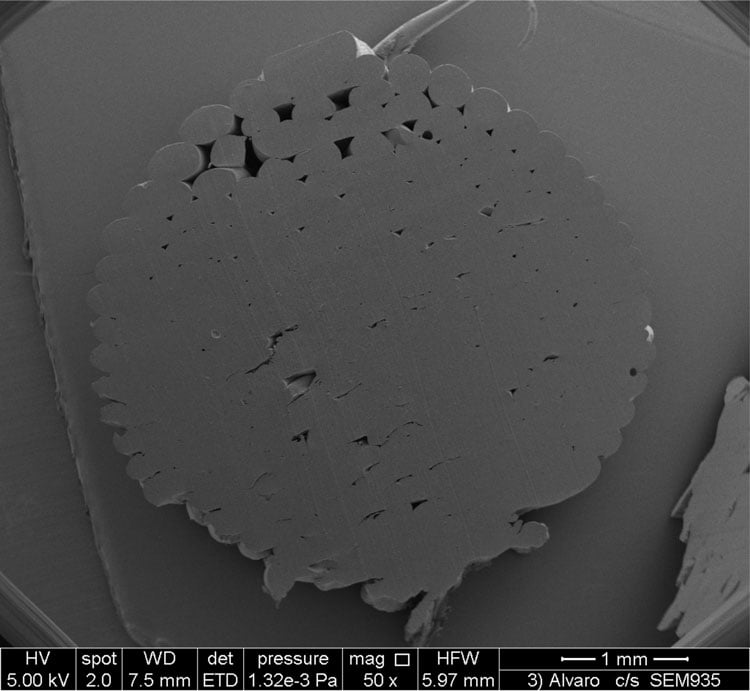

Figure 1: Cross-section of a printed dosage form, showing the individual polymer strands that adhere to form the final object
Complex or multilayer tablets are also easily fabricated with FDM printing3 (see Figure 2). This is because FDM printers are easily constructed with multiple print heads, and so it is possible to print objects from different filaments. The filaments can be comprised of different polymers, for fabrication of modified or sustained-release devices, or may contain different drugs, allowing for the manufacture of multi-drug polypills. For instance, Figure 3 () shows Raman images of a polypill containing caffeine and paracetamol3. Each drug was encapsulated in a separate polymer filament, and the tablet was printed with a dual-head FDM printer. The Raman images are colour-coded to show the locations of the two drugs and it is clearly evident that the drugs remain in separate layers after printing.
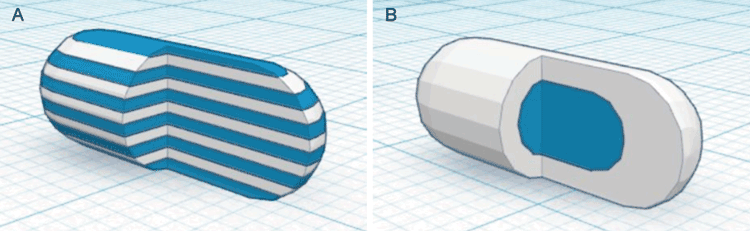

Figure 2: 3D representations of heterogeneous printed solid dosage forms: (A) sectioned multilayer device and (B) sectioned DuoCaplet (caplet in caplet)
One drawback of FDM printers is that the printing temperature is dependent on the softening or melting temperature of the polymer from which the filament is constructed. If the drug to be printed is photolabile, there is a risk of significant thermal degradation as the material passes through the print head. This issue may be ameliorated to a degree by careful blending of the polymers used to make the filament, or by adding plasticisers, to lower the printing temperature. It may also be possible to increase the speed of printing, to reduce the time the drug is exposed to heat. However, careful thought is needed when considering using FDM printing to formulate thermally labile drugs.
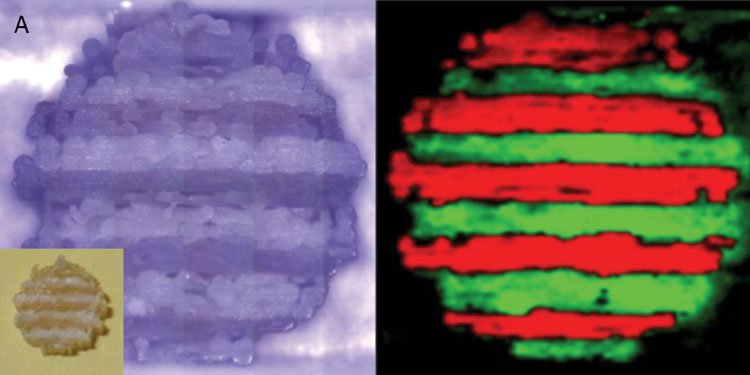

Figure 3: Raman microscopy images of a polypill printed with an FDM printer. The image on the left is the original image from the microscope (inset shows the tablet itself) and the image on the right is colour coded to show the location of the drugs (caffeine in green, paracetamol in red)
It is also possible to use 3D printers to deposit gels and semi-solids. In this case, the print head is essentially a syringe which is driven by a motor. The semi-solid material is extruded from the syringe by the motor, and moving the print head in a raster pattern across a build plate builds up the object, in a similar fashion to the FDM printers just described. Printing a semi-solid material requires careful formulation, because the material must be viscous enough to flow, but must remain in place on the build plate after deposition. This may be accomplished by strategies such as developing thixotropic systems or using a heated build plate to dehydrate or ‘set’ the mixture.
The final type of 3D printer is stereolithographic (SLA). In this case, the feedstock is a resin monomer and the print head is a laser. The laser is focussed to a specific depth in the resin tank and the wavelength is selected so that excitation of the resin monomers with laser light causes photo-polymerisation. Then, as in the cases described, the laser is moved in a raster pattern to create one layer of the object, before the build plate is moved and the process repeats. If the excitation wavelength of the laser is not exactly matched to the resin, a photo-initiator can be added.
SLA printers have some unique advantages that favour production of pharmaceuticals. Firstly, they do not require elevated printing temperatures (although there can be local heating as the polymer polymerises), and so have more potential for thermally labile drugs. Also, drugs with poor aqueous solubility may naturally dissolve to a higher concentration in the resins, and so be easier to formulate at high dose (in cases where drug solubility in the resin is poor, drug particles may be suspended in the resin). Where the resin used is miscible or soluble in water, it is possible to encapsulate water in the printed object, and so print drug-loaded hydrogels directly.
One drawback of SLA printing is that the resin monomer (which are typically polyethylene glycol diacrylates) and photo-initiator may have toxicity issues and so work is needed to develop curable pharmaceutical materials. The technique does, however, look very promising as a manufacturing technology for making implantable, drug-loaded devices.
The role of 3DP in pharmaceutical manufacturing
The current applications of 3DP to pharmaceuticals are at an early stage, and all the types of printer described herein need properly developed feedstock, comprised of pharmaceutical-grade materials. The powder-bed system has clear potential in light of the recent first FDA-approved 3DP tablet and naturally lends itself to agglomerated powder systems. Feedstock filaments for FDM printers are being developed and it is surely only a matter of time before filaments of pharmaceutical-grade polymers are developed. Polymeric printed dosage forms lend themselves to targeted delivery, either through careful control of shape, selection of pH-sensitive enteric polymers or fabrication of multi-layered polypills. SLA printers have potential in the areas of hydrogels and drug-loaded implantable devices.
Selection of the drugs to be formulated will remain a critical issue. There has not, as of yet, been a drive to replace commercial batch processes, such as powder compaction or capsule filling, with 3DP technology. And so the immediate areas of application lie, for instance, in drugs that (i) are highly-potent and so have a low dose and/or narrow therapeutic index, (ii) can be formulated as an oro-dispersible tablet, in which case 3DP technology might be economically favoured over manufacturing routes involving freeze-drying, (iii) need to be incorporated into implantable devices manufactured to contour-specific patients and (iv) are biopharmaceuticals, and so have a limited number of patients.
It is relatively easy to envisage how 3DP may alter the pharmaceutical manufacturing landscape, if appropriately developed over the next few decades. In the short term, more printed dosage forms will be approved, probably designed as fast-dissolving or controlled-release. Over the longer term, the basic technology will reduce further in price and will be optimised for pharmaceutical manufacture, reducing the fabrication time of unit doses to a few seconds. The printers could be situated in pharmacies, while the feedstock materials could be commercially produced. The pharmacist could then extemporaneously print a supply of tablets, with the dose and/or dose combination tailored to the patient. For biopharmaceutical products, it can be imagined that a biopharmaceutical might be prepared from a patient’s blood sample, and formulated into a printed form in a continuous process – truly ‘printing a dose of one’s own medicine’.
References
- Goyanes A, Buanz ABM, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014; DOI:10.1016/j.ijpharm.2014.09.044
- Goyanes A, Robles Martinez P, Buanz ABM, Basit A, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm., 2015, DOI: 10.1016/j.ijpharm.2015.04.069
- Goyanes A, Wang J, Buanz A, Martinez-Pacheco R, Telford R, Gaisford S, Basit A. 3D printing of medicines: Engineering novel oral devices with unique design and drug release characteristics. Mol. Pharm., 2015, DOI:1021/acs.molpharmaceut.5b00510
Biography
Dr. Gaisford is a Reader and Head of Department of Pharmaceutics at UCL School of Pharmacy, University College London. With Professor Abdul Basit and Drs. Steve Hilton and Alvaro Goyanes, he leads a team pioneering the use of ink-jet and 3D printing in fabricating pharmaceutical dosage forms and has established a company, FabRx Ltd, to commercialise the work. He has published 80 papers, three books and six book chapters. Contact Dr. Gaisford at: s.gaisford@ucl.ac.uk (Tel: +44(0)20 7753 5863. Fax: +44(0)20 7753 5942)
Related topics
3D printing, Personalised Medicine
Related organisations
University College London School of Pharmacy
Related people
Dr Simon Gaisford


