Flow cytometry: breaking bottlenecks in drug discovery and development
Posted: 20 September 2016 | Pooja Sharma, Pushpanathan Muthuirulan | No comments yet
The discovery and development of novel drug candidates is a highly complex, time-consuming and expensive process. Pharmaceutical scientists and clinicians should, therefore, reevaluate and modify the existing standard platforms and increase their uptake of robust advanced technologies such as flow cytometry to overcome the bottlenecks in these processes…

The discovery and development of novel drug candidates is a highly complex, time-consuming and expensive process. Pharmaceutical scientists and clinicians should, therefore, reevaluate and modify the existing standard platforms and increase their uptake of robust advanced technologies such as flow cytometry to overcome the bottlenecks in these processes. A wide variety of flow cytometry methods have been employed at different stages of drug discovery and development. With the implementation of multi-parameter intracellular flow cytometric analysis at high-throughput single-cell resolution, rapid drug molecule screening is enabled; drug targets identified; complex interrelated mechanisms of drug action elucidated; medicines’ off-target effects characterised; and drug safety, pharmacokinetics and pharmacodynamic effects assessed. This article explores the benefits and challenges associated with unique applications of flow cytometry in various stages of drug discovery and development.
Introduction
Flow cytometry presents an attractive prospect for drug discovery and development pathways due to its exceptional capacity to analyse heterogeneous populations of cells. It provides insights into the multiparameter functional and biological information of a single cell at higher resolution. Additionally, continued advancements in flow cytometry methods such as high-throughput multifactorial analysis; improvement in cell sorting; and rapid event detection and resolution guarantees enhanced efficiency in identifying and characterising novel bioactive drugs1.
New areas of application continually arise in flow cytometry, adding to the range of already highly specialised techniques for performing cell-based assays in drug discovery, and there is also an expanding role for flow cytometry in evaluating drug toxicity. Furthermore, the development of flow cytometric bead array technologies has opened up new avenues of research that provide insights into metabolic or disease biomarkers, cellular signalling, immune regulatory mechanisms, growth factor biology, isotyping, neurobiology and transcription factors/nuclear receptors, which have all added benefits to the use of flow cytometry in drug discovery and development processes2 .
Drug Target Review has just announced the launch of its NEW and EXCLUSIVE report examining the evolution of AI and informatics in drug discovery and development.
In this 63 page in-depth report, experts and researchers explore the key benefits of AI and informatics processes, reveal where the challenges lie for the implementation of AI and how they see the use of these technologies streamlining workflows in the future.
Also featured are exclusive interviews with leading scientists from AstraZeneca, Auransa, PolarisQB and Chalmers University of Technology.
Flow cytometry in biomarker discovery
The combination of multi-parameter phenotypic profiling and cellcentric technologies have fostered the application of flow cytometry in novel drug molecule screening and its optimisation for use in clinics – especially in cancer therapeutics3 . Over the past decade, biomarkers have become instrumental and demonstrated ‘proof of concept’ to improve the effectiveness and efficiency of decision-making processes in lead identification and characterisation. The continuous discovery of novel biomarkers and development of new technologies to measure biomarkers have provided new paths in employing flow cytometry for the characterisation of drug target effects, which plays a central role in generating new personalised approaches for treating patients with safe drugs4 .
High-throughput flow cytometry in small-molecule discovery
The advent of high-throughput (HT) flow cytometry signalled a new era for biomedical research. It uses plug flow technology, which employs a peristaltic pump, auto sampler and microwell plates to boost the assay throughput5 . Furthermore, the integration of multicolour technology has enabled multiplexing and homogenous discrimination of ligand binding and assembly of macromolecules. HT flow cytometry has extended applications in cell-based assays such as cell proliferation, differentiation, cell death, adhesion, ligand binding, transport and cellular signalling. It has been used widely for a variety of purposes, such as HT screening (HTS) assays for ligands of the formyl peptide receptor (fpr); GPR30 and estrogen receptors; disassembly of the 26S proteasome; quorum sensing inhibition; screening to identify small-molecule inhibitors for ABC transporters; antifungal efflux system and Ras/ Ras-related GTPase6 .
Evaluating drug effect
During recent years, the use of cell culture systems to study the pharmacological effect of drugs has become more practical, convenient and cost effective. The health of the cultured cells is a crucial factor to be considered when using flow cytometry to study drug-cell interactions. Using unhealthy cells promotes variability in drug-induced cell responses that may provide inconsistent or unreliable sources for drug validation. Flow cytometry is a dynamic approach that offers the best routes for sequential evaluation of drug-induced responses in cultured cells at specific time points. In this context, flow cytometry has demonstrated great value in understanding cell-population distributions, which gives insight into drug-induced changes in cell cycle events such as cell proliferation arrest and apoptosis7 .
Flow cytometry presents an attractive prospect for drug discovery and development pathways due to its exceptional capacity to analyse heterogeneous populations of cells
Flow cytometry uses biomarkers to evaluate cell-cycle progression in growing cell populations. This analysis in growing cells, which is based on biosynthetic incorporation of thymidine analogues BrdU or EdU into newly-synthesised DNA and evaluation of cell cycle proteins (cyclins), can provide a wealth of information on drug molecules that interfere with the progression of cell proliferation8,9. Furthermore, druginduced apoptosis in target cells can be evaluated by flow cytometry through the analysis of a series of events, such as phosphatidyl serine exposure on apoptotic cell membranes, release of intracellular cytochrome c/caspase 3 and DNA damage10. Thus, the use of flow cytometry-based approaches to measure drug-induced proliferating activity and apoptosis in the same cell at different time points can provide a broader understanding of mechanisms of drug action and it also honours the concept of using flow cytometry as a standard tool for oncology drug discovery.
Drug safety assessment
Applying flow cytometry to drug safety assessment (that is, preclinical toxicity studies) has brought multiple benefits to biomedical research, since it offers an attractive tool to identify and characterise druginduced toxicity using animal models (in vivo) and cell culture systems (in vitro). Flow cytometry methods enable acute (or) subacute (or) chronic toxicity to be measured, as well as the carcinogenic, mutagenic and teratogenic effects in tissue samples or cultured cells to be assessed11. The feasibility of preparing single-cell suspensions of tissue samples by homogenisation or dissociation protocols makes flow cytometry methods more amenable for preclinical toxicity testing of drugs on any tissue samples. Most drug molecules can induce toxicity by altering the Redox status within the cells. Therefore, flow cytometry-based measurement of biomarkers for drug-induced oxidative stress including lipid peroxidation, mitochondrial membrane potential, loss of plasma membrane integrity, intracellular glutathione and phosphatidyl serine exposure are highly instrumental in assessing the toxic nature of drug molecules12.
Implementing flow cytometry into drug safety assessment studies has improved the robustness of assays, conferred strong statistical data, reduced time, increased cost effectiveness and utilised fewer resources, which have all added value to the pharmaceutical industry as well as clinical laboratories.
Evaluating drug efficacy
The translational medicine approach has been increasingly used in clinical drug development pathways to provide drug molecules that are safer and more efficacious to use. Drug efficacy can be evaluated by analysing the cellular components of immune systems (pharmaco – dynamics [PD] biomarkers), which can potentially bridge the gap between drug discovery and development pathways. Flow cytometry represents the most efficient tool with which to analyse PD biomarkers such as cell proliferation markers, cytokine production and modulation of cell surface markers, with relatively high throughput and greater precision, which may be helpful in evaluating the efficacy of the drug molecule for its use in clinics13.
Besides these, the flow cytometry-based evaluation of PD biomarkers in the healthcare setting also facilitates the character – isation of abnormalities of cellular components of the immune system associated with autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis (RA), Crohn’s disease and systemic sclerosis14. Therefore, the widespread application of flow cytometry in monitoring cellular components of the immune system can provide insights into risk factors associated with autoimmune diseases and also facilitate clinical drug development processes.
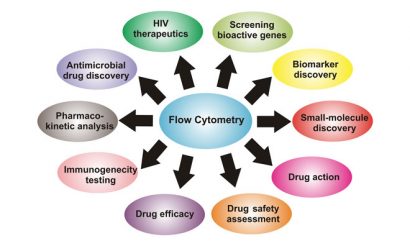

Figure 1: Versatile application of flow cytometry in drug discovery and development process
Immunogenicity testing and pharmacokinetic analysis
The use of flow cytometry in immunogenicity testing and pharmacokinetic analysis offers the advantage of enabling cell viability based on changes in growth characteristics of cell populations to be assessed, as well enabling drug quantification analysis, for use in preclinical or clinical studies. Immunogenicity assays using flow cytometry can investigate drug binding to cell surface receptors and downstream activation of signalling mechanisms (i.e., protein phosphorylation, cAMP production and hydrolysis of inositol phosphate) in response to drug simulations15. Pharmacokinetic studies of drugs using flow cytometry can be performed using cell lines with naturally expressed antigens or engineered cell lines with homogenous and high surface antigen expression. An examination of antigen shedding or modulation (internalisation) in response to drug stimulation can provide reliable pharmacokinetic assessment of drugs16.
HIV therapeutics
The use of flow cytometry in evaluating CD4+ T-cells has helped address the technological challenges associated with HIV therapeutics. CD4 has proven to be an important prognostic marker to assess HIV progression, drug resistance and host susceptibility to secondary infections by opportunistic pathogens, and so accurately monitoring the levels of CD4+ T-cells in HIV patients via flow cytometry can aid in developing effective antiretroviral (ARV) therapies17. Also, the combined application of lymphocyte counts from a haematology analyser and CD4 percentage value from flow cytometry can effectively determine the variability in CD4 absolute values to ensure that the patients receive the most effective retroviral treatments18.
Screening bioactive genes
Flow cytometry coupled with metagenomics approaches sheds light on new high-throughput metagenome screening strategies, known as SIGEX and PIGEX (substrate/product induced gene expression profiling), which has led to numerous bioactive genes from environmental sources being identified19,20. These strategies have several advantages in metagenome screening, which offer an efficient way for high-throughput screening of bioactive molecules to take place, since it allows automated screening of metagenome-derived libraries using flow cytometry that reduces time, labour and expenses in identifying a novel drug candidate. Therefore, these strategies may be helpful in the initial screening and identification of novel drug molecules for its subsequent characterisation and clinical development.
Peptide-based antimicrobial drug discovery
Flow cytometry – with its ability to discriminate and quantify multiple parameters of target cells – has particular use in classifying and characterising novel antimicrobial peptides (AMPs). AMPs are broadly classified into membrane-acting or cell-translocating types. Membrane-acting AMPs form transient pores on the microbial membranes that cause leakage of intracellular contents leading to cell death, whereas cell-translocating AMPs can translocate across microbial cell membranes and exhibit their antimicrobial activities by inhibiting essential cellular processes.
Fluorescently labelling AMPs, such as with fluorescein isothio – cyanate, followed by assessing their internalisation into microbial cells by flow cytometry, can efficiently distinguish membrane-acting from cell-translocating AMPs. Further, use of FITC-dextran of different molecular masses in the assay coupled to flow cytometry analysis can measure the extent of the membrane damage caused by AMPs. Cell-translocating AMPs exhibit a spectrum of activities and exert antimicrobial actions in the following ways: (i) by inducing endogenous accumulation of reactive oxygen species (ROS); (ii) by inhibiting DNA synthesis; (iii) via transcription inhibition; (iv) by inducing DNA damage; or (v) by disrupting the mitochondrial membrane potential.
These mechanisms can be measured using flow cytometry by staining AMP-treated cells with fluorophores specific for different modes of action such as 2′,7′-dichlorodihydrofluorescein diacetate (for ROS detection), ethynyl-deoxyuridine (inhibition of DNA synthesis), 5-ethynyl uridine (inhibition of transcription), TUNEL reagents (DNA damage) or rhodamine123 (mitochondrial membrane potential)21. Thus, flow cytometry offers a suitable framework for understanding distinct classes of AMPs and also provides insights into their different mechanism of actions, which has a notable impact on prospective antimicrobial drug development.
Conclusion
To conclude, flow cytometry is one of the most powerful tools for the drug discovery and development life cycle. It is highly affordable, speedy, easy to use, accessible to even those without expertise and provides accurate quantitative data for most essential cellular assays. This enables rapid drug molecule screening and optimisation for use in clinics. In addition, recent advancement in flow cytometry offers new and exciting approaches that provide versatile platforms with applications at all phases of drug discovery and development (see Figure 1).




Related topics
Antimicrobials, Flow Cytometry
Related organisations
Maryland University, National Institutes of Health (NIH)
Related people
Pooja Sharma, Pushpanathan Muthuirulan



