Protein receptor linked to cell death imaged for the first time
Posted: 4 October 2019 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have imaged an inflammation-related protein receptor on cell membranes which could inform future drug designs to prevent cell death.
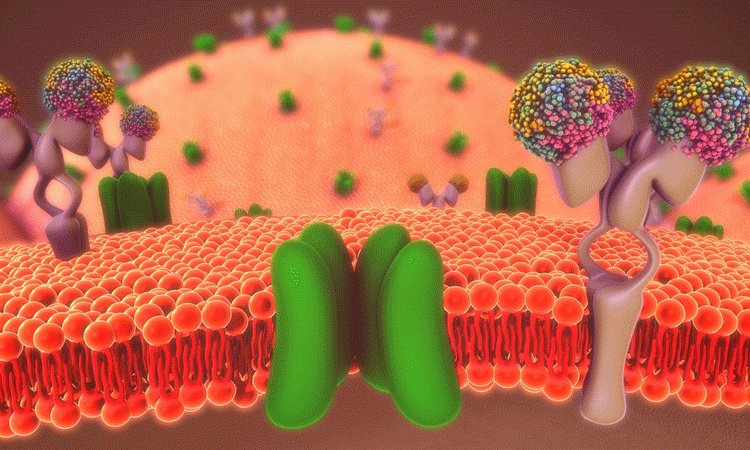

Researchers have for the first time imaged a protein that is associated with numerous health problems. They suggest that their findings could help to design drugs to combat inflammation, coronary artery disease, cancer, multiple sclerosis and others.
The study was conducted at the Oregon Health & Science University (OHSU), US. The team investigated a protein receptor on the cellular membrane which allows electronically charged sodium and calcium particles to enter and cause changes in cells.
Using cyroelectron microscopy, the researchers captured the three-dimensional (3D) structure of the P2X7 protein receptor and observed its inner workings. The receptor is a subtype of the ligand-gated ion channel P2X family.
Previous studies have found that once the P2X7 receptor is activated, its channel remains open indefinitely, continually allowing charged particles to enter a cell and trigger the signalling pathways for inflammation, ultimately leading to cell death.


This illustration shows how the P2X7 protein receptor opens and closes, allowing charged particles to enter a cell and trigger cellular changes. OHSU researchers used cryoelectron microscopy to obtain the protein’s 3D structure for the first time. P2X7 is associated with many diseases, including inflammation, coronary artery disease, cancer and multiple sclerosis (credit: Oregon Health & Science University).
The scientists were able to visualise the parts of the receptor that sit inside the cell. As a result, they were able to directly observe how these are modified with fatty acid molecules called palmitoyl groups.
Upon removing these groups, the researchers found that the receptor did not continue to stay open. They also discovered a guanosine nucleotide that was bound to P2X7 inside the cell.
“Researchers have known ligand-gated ion channels are modified by palmitoyl groups, but we had never directly observed it until now,” said Steven Mansoor, an assistant professor of medicine at OHSU. “Our finding could be used as a model of how palmitoylation modifies other ion channels.”
The team plan to further examine the role of these palmitoyl groups and the guanosine nucleotide play in P2X7 intra-cellular signalling and how they could be targeted to treat related health conditions.
The results were published in Cell.
Related topics
Drug Discovery, Drug Targets, Microscopy, Protein, Research & Development
Related conditions
Cancer, Coronary artery disease, inflammation, Multiple Sclerosis
Related organisations
Oregon Health & Science University
Related people
Assistant Professor Steven Mansoor



