AbbVie and Bristol-Myers Squibb announce lung cancer clinical trials collaboration
Posted: 25 July 2016 | Niamh Louise Marriott, Digital Content Producer | No comments yet
AbbVie and Bristol-Myers Squibb Company today announced a clinical trial collaboration to evaluate the safety, tolerability and efficacy of AbbVie’s investigational biomarker-specific antibody drug conjugate Rova-T…
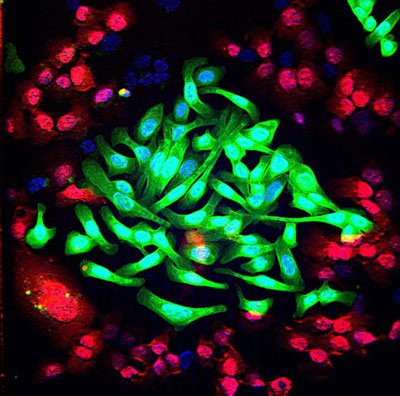

Lung cancer cells (green) are cultured together with normal lung cells (red). The triple-antibody combination EGFR, HER2 and HER3 strongly impairs the survival of tumor cells while sparing normal cells. Modified confocal microscopy image: Maicol Mancini, lab of Professor Yosef Yarden. CREDIT: Weizmann Institute of Science.
AbbVie and Bristol-Myers Squibb will collaborate in a clinical trial to evaluate the safety, tolerability and efficacy of AbbVie’s investigational biomarker-specific antibody drug conjugate Rova-T (rovalpituuzumab tesirine) in combination with Bristol-Myers Squibb’s Opdivo (nivolumab) as a treatment for relapsed extensive-stage small cell lung cancer (SCLC).
Treatment options
Small cell lung cancer is a difficult-to-treat form of cancer that accounts for approximately 15 percent of all lung cancers. The five-year survival rate for extensive-stage SCLC is less than 5 percent and treatment options are limited for the more than 234,000 people diagnosed annually.
Phase I and II clinical trials planned
The Phase I/II clinical program will explore the potential of combining Bristol-Myers Squibb’s immuno-oncology agents, which are designed to alleviate immune suppression, in conjunction with AbbVie’s investigational antibody drug conjugate, Rova-T.
Rova-T is an antibody drug conjugate that targets and eliminates tumour initiating cells and other bulk tumour cells. This collaboration will determine if the targeted cell killing and antigen release caused by Rova-T may further enhance the effect of immunotherapy.
“We are excited to explore the potential benefits of combining Bristol-Myers Squibb’s immunotherapies with a targeted approach like Rova-T in small cell lung cancer where the need for new therapies is particularly acute for this aggressive form of lung cancer,” said Jean Viallet, M.D., Global Clinical Research Lead, Oncology, Bristol-Myers Squibb.
“As the science around cancer research continues to rapidly evolve, we are building on our leadership in Immuno-Oncology with numerous collaborations that may help advance new therapies for cancers in need of better options.”
Scott J. Dylla, Ph.D., VP of research and development, AbbVie said, “by combining immune-checkpoint inhibitors that prime the body’s immune system to fight cancer cells with Rova-T’s approach to target cancer stem cells, we hope to build on our goal to develop differentiated treatments with therapeutic benefit that elevate the standard of care for small cell lung cancer patients.”
The first line of defence?
Rova-T is a biomarker-specific therapy that targets cancer stem cells and combines a targeted antibody that delivers a cytotoxic agent directly to cancer cells expressing a delta-like protein 3 (DLL3). Rova-T is currently in investigational studies as a third-line treatment for SCLC but AbbVie will initiate a first-line clinical study for Rova-T in SCLC and several other types of tumours in the near term.
Related topics
Stem Cells
Related conditions
Lung cancer
Related organisations
AbbVie, Bristol-Myers Squibb



