Synthetic macrocyclic receptors as tools in drug delivery and drug discovery
Posted: 10 October 2014 | Amir Norouzy (Jacobs University Bremen), Werner Nau (Jacobs University Bremen)
Macrocyclic compounds (see Figure 1) have fascinated medicinal chemists for generations. The first ones which have been characterised are the naturally occurring cyclodextrins (CDs), cyclic oligomers of glucose, which can be obtained by bacterial synthesis from starch. Calixarenes (CXs) were among the first man-made macrocycles, followed by crown ethers (Pedersen, Nobel Prize 1987) and cucurbiturils (CBs). While initially challenges related to their efficient synthesis and derivatisation needed to be tackled, their functions as molecular receptors have subsequently moved into the focus, which is the basis for the pharmaceutical and medicinal-diagnostic applications summarised in the following article.

The depicted CDs, CXs, and CBs have their highly water-soluble nature in common because they are laced by hydrophilic groups at their top and bottom rims but they possess a hydrophobic, cone- or barrel-shaped cavity predestined for the encapsulation of suitably sized hydrophobic guests. It is important to note that these types of macrocycles are available in different cavity dimensions, that is, different homologues with at least four and up to more than 10 monomeric repeat units can be synthesised. Note that macrocyclic receptors are superior to flexible acyclic ones, because their shape is pre-organised for the formation of a so-called ‘host-guest’ complex, nicely fulfilling the requirement for Fischer’s lock-key principle. Not surprisingly, macrocycles are considered as mimics of enzymatic binding pockets or biological receptors. This biomimetic receptor functionality, along with their biocompatibility1-3, make them suitable for a large range of biological and medicinal applications which range from drug delivery to drug discovery described herein.
Drug delivery
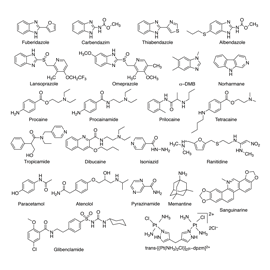
Owing to the intrinsic water solubility of many macrocycles, their resulting host-guest complexes with different pharmaceutical drugs retain also a high water solubility. Moreover, the encapsulated drugs have different pKa values than the uncomplexed ones4-6. In particular, the guest molecules can become protonated when immersed in CXs or CBs, which greatly assists their solubilisation. This is of particular interest when the drug themselves have an insufficient water solubility. In these cases, macrocycles can be used to solubilise drugs7,8, e.g., when intravenous administration is desirable. To illustrate, the solubility of several benzimidazole-based anthelmintic and fungicides drugs increases by up to a factor of 100 upon encapsulation by CBs9. In contrast to liposomal or polymeric drug formulations, macrocyclic ones constitute discrete, supramolecular systems, whose pharmacokinetic properties can be readily predictively tuned, for example, to achieve a slow, sustained release. As an additional advantage, due to the spatial isolation of the encapsulated drug from the aqueous environment, enzymatic and other chemical degradation reactions can be effectively suppressed, in part assisted by their different protonation state10. This feature is already routinely exploited commercially in CD-based drug formulations, e.g., for oral testosterone delivery.
The rest of this article is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related topics
Biomarkers, Drug Delivery, Drug Discovery, Drug Targets, Enzymes
Related organisations
Jacobs University Bremen
Related people
Amir Norouzy, Werner Nau



